Introduction
Once the diffusion of oxygen occurs across the alveoli, it is transported to the tissues for unloading through the bloodstream, while carbon dioxide diffusion through the alveoli out of the bloodstream follows for expulsion out of the body. Oxygen and carbon dioxide transportation occurs through different mechanisms.
Transport of Oxygen in the Blood.
Only a small portion (1.5
Hemoglobin.
Hemoglobin (Hb), is a protein located in red blood cells (erythrocytes) made of four subunits, namely: two beta subunits and two alpha subunits.
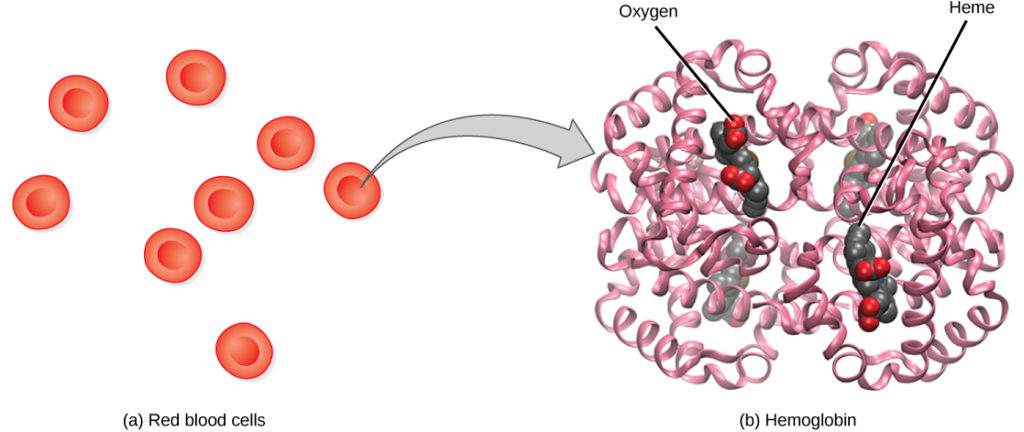
The heme group is surrounded by each of the four subunits and contains iron and binds a single oxygen molecule; therefore, each hemoglobin molecule binds four oxygen molecules. Molecules that have more oxygen bound to the heme groups appear brighter red, a condition that explains why oxygenated arterial blood where the hemoglobin carries four oxygen molecules looks brighter red compared to deoxygenated venous blood which appears darker red.
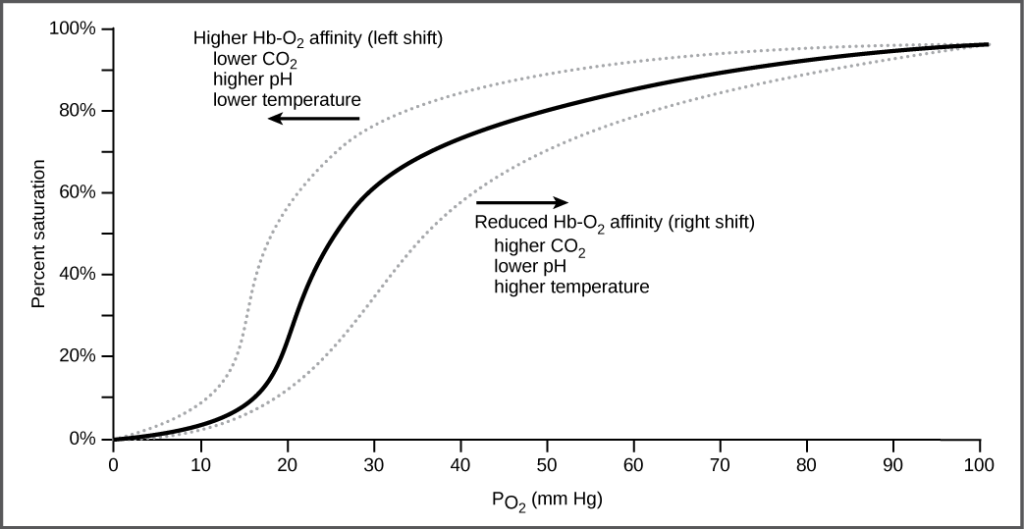
As oxygen binds to the hemoglobin, it changes its shape hence the binding of the second and third oxygen molecule is easier than the first one. The fourth oxygen molecule is then even more difficult to bind.
The oxygen dissociation curve is sigmoidal or S-shaped. This is because as the partial oxygen pressure increases, the hemoglobin is increasingly saturated with oxygen.
Transport of Carbon Dioxide in the Blood.
Transfer of carbon dioxide in the blood from the body tissues to the lungs can be achieved through three methods.
- Binding to the hemoglobin
- The direct dissolution into the blood.
- Transportation as a bicarbonate ion.
Properties affecting carbon dioxide transport.
- Carbon dioxide is more soluble in blood compared to oxygen, whereby 5-7
- Carbon dioxide binds to plasma proteins or enters red blood cells to bind on to hemoglobin, an activity which transports about 10
- The majority (85
CO2+H2O⟷H2CO3(carbonic acid)⟷HCO3+H+(bicarbonate)
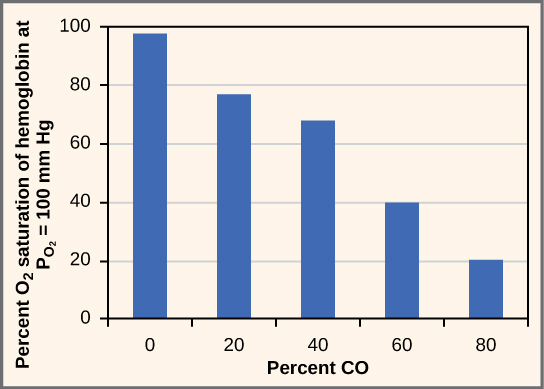
During expiration, hydrogen ions dissociate from hemoglobin to bind to the bicarbonate ion which the carbonic acid intermediate. This is followed by the conversion of the intermediate back into carbon dioxide by CA enzymatic action. The produced carbon dioxide is expelled through the lungs.