When water is taken in an exceedingly glass vessel, the free surface of the water close to the walls is curved concave upward. If mercury is extracted in a glass vessel, then the free surface of mercury near the walls is convex upwards.
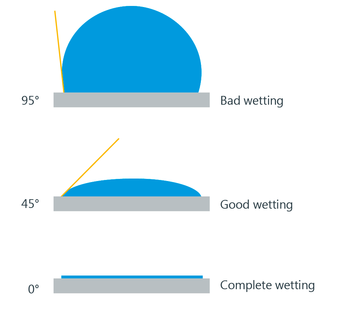
When the liquid is connected with solid, the angle between the solid surface and also the tangent to the free surface of the liquid at the purpose of contact, measured from within the liquids called the angle of contact. The angle of contact is most acute when the liquid surface is curved concave. When the liquid surface is curved convex upwards, the angle of contact is obtuse.
Characteristics of the Angle of Contact
- For a given liquid-solid pair, the angle of contact is constant.
- When the angle of contact between the liquid and a solid surface is tiny (acute), the liquid is said to wet the surface. Thus water wets glass.
- If the angle of contact is massive the surface isn’t wetted. Mercury does not wet glass.
- If there are impurities found in liquid, then they change the values of the angle of contact.
- The angle of contact constantly decreases when there is an increase in temperature.
- For a liquid that utterly wets the solid, the angle of contact is equal to zero.
Acute Angle of Contact
When impure water or kerosene is taken in an exceedingly glass vessel, it is found that the surface near the walls is curved concave upwards.
Consider a molecule of water M on the free surface very close to the wall of the glass vessel. The force of cohesion C because of different water molecules is as shown within the figure. In addition to the current, a force of adhesion A acts due to the glass molecule as shown in the figure.
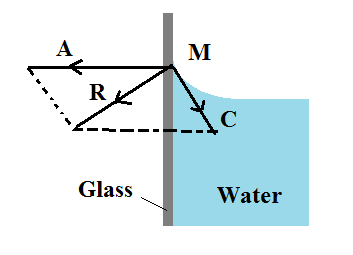
The net adhesive force between water molecules and air molecules is negligible. The gravitational force on the molecule is also negligible. The immensity of A is greater than the magnitude of C and the aftermath of the two molecular forces of attraction R is conducted towards the glass or outside the liquid. Hence, the walls of the glass vessel attract A molecule. The surface of water that is free always adjusts itself at right angles to the resultant R. So the molecules like M edge upward on the solid surface. Thus the water surface is curved concave upwards and also the angle of contact is acute.
Obtuse Angle of Contact
Whenever mercury is extracted in a glass vessel, it is discovered that the surface near the walls is curved convex upwards. Consider a molecule of mercury M on the free surface very close to the wall of the glass vessel. The force of cohesion C because of different mercury molecules is as shown within the figure. In addition to this, a force of adhesion A acts because of the glass molecule.
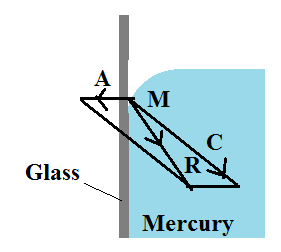
The net adhesive force between mercury molecules and air molecules is negligible. The gravitational force on the molecule is also negligible. The magnitude of A is very less than the magnitude of C and resultant of the two molecular forces of attraction R is directed within the liquid. Hence the molecule A is attracted towards other molecules of mercury. The free surface always adjusts itself at perfectly right angles to the resultant R. The molecule A slides downwards on the glass surface. Thus the surface of the mercury within the glass is curved convex upwards and the angle of contact is obtuse.