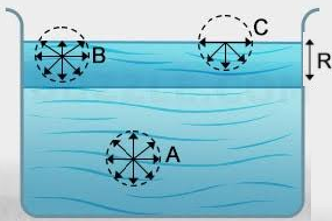
Mosquitoes sitting on the water
In rainy reason, diseases like dengue and malaria spread by mosquito breeding on fresh stagnant water. They do not sink in water because of surface tension. At the points wherever the legs of the mosquito touch the liquid surface, the surface becomes concave due to the weight of the mosquito.
The surface tension acting tangentially on the free surface acts at a precise angle to the horizontal. Its vertical component acts upwards. The total force acting vertically upwards right along the road of contact of certain length balances the load of the mosquito acting vertically downward.
Excess Pressure on Concave Aspect of a Spherical Surface
The surface tension on the two sides tangential to the surface balances when the surface is plane and then the resultant tangential force is probably going to be zero.
However, in the case of the surface being convex or concave, the forces in the course of surface tension which are acting across the sides will have resultant force towards the center of curvature of the surface. Thus, whenever the surface is arched, the surface tension gives rise to a pressure directed towards the center of the curvature of the surface.
This pressure is balanced by an equal and opposite pressure functioning on the surface. Therefore, there’s continuously an excess pressure on the concave side of the arching liquid surface.
Detergents and Surface Tension
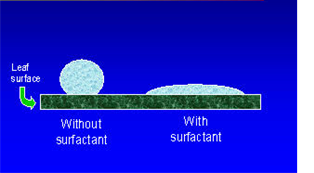
Detergents can remove oil stains from clothes. Water is used as a cleaning agent. The surface tension of water is lower because of soap and detergents.
This is desirable for laundry and cleansing since high surface tension of pure water doesn’t permit it to penetrate easily between the fibers of materials, where dirt particles or oil molecules are held up. The surface tension of soap solution is smaller than that of pure water however the surface tension of detergent solutions is smaller than that of soap solution.
That is why detergents are more practical than soap. A detergent dissolved in water loosens the hold of dirt particles on the fabric fibers that get easily detached when the fabric is squeezed.
Wax-Duck Floating on Water
The surface tension of liquids decreases because of dissolved impurities. If you stick a pill of camphor to the rock bottom of a wax-duck and float it on still water surface, you will observe that it begins to move randomly after a minute or two.
This is as a result of camphor dissolves in water and therefore the surface tension of water just under the duck becomes smaller than the encompassing liquid. This creates a net difference of force of surface tension that makes the duck maneuver.
Drop-Based Concepts of Surface Tension Measurement
One of the concepts is based on the analysis of a drop. This analysis can be:
A complex optical analysis of the drop shape,
Related to the force required to make a bubble burst, or,
It depends on the time it takes a group of drops to traverse a tube of small diameter.
These methods acquire advanced mathematical analysis. Also, they are hard to perform on your own.