The isothermal process is the thermodynamic process, in which the temperature of the system remains constant. The heat transfers out of the system or to the system should happen at the slower rates for continuous adjustment to the temperature of the reservoir, through the process of the heat exchange. The thermal equilibrium is maintained in all of these states. Whereas, the adiabatic processes are also thermodynamic processes but there is no transfer of heat into or out of the system. In the adiabatic processes, the energy transfer can be seen only as work. However, the assumption of no transfer of heat is very important.
Comparison Between Isothermal and Adiabatic Processes
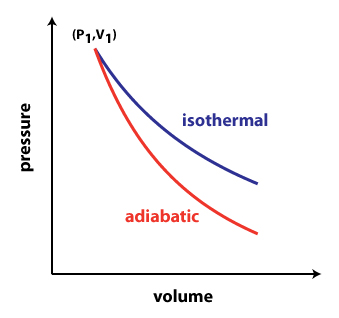
Both the isothermal process and the adiabatic process are commonly used in terms of thermodynamics during consideration of energy variation in the form of heat. For understanding the difference between the isothermal and the adiabatic processes one can get a clear understanding by using the Carnot heat engine. The Carnot heat engine is used for describing the virtual model of the thermodynamic system in which the exothermic and endothermic processes are performed. A thermodynamic system gives the heat from the hot reservoir and then this heat sinks to the cold reservoir. In the meantime, the four processes such as isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression are continuous.
Isothermal Processes and the State of Matter
There are many isothermal processes and they are greatly varied. One of the most common examples is the evaporation of the water to the air. Many chemical reactions are responsible for maintenance of the thermal equilibrium and in biology, the interaction of the cell with the surrounding cells is also considered as the isothermal process. Melting, boiling, and evaporation are also known as phase changes and they are taking place at the constant pressure and temperature. In physics, the isothermal process can be charted by following the vertical line along with the constant temperature.
Isothermal Process vs Adiabatic Process
Adiabatic Process and Time Scale
A longer time is required for the adiabatic processes as in the mechanical processes the smaller time scales renders the adiabatic process impossible. However, the minute transfer of the heat over the system boundaries can be revealed by the smaller time scales, and ultimately, the course of work will be balanced. The factors such as the rate of heat dissipation, amount of the work done, and the amount of heat lost due to the imperfect insulation can significantly affect the outcomes of the heat transfer in the overall process.