Some gases are made up of molecules. What are molecules? Molecules are a tiny independent unit that behaves the same as the sample, i.e. they have the same chemical properties as of the sample. The kinetic theory of gases has developed a model that explains the behavior of molecules, which should further explain the behavior of an ideal gas. This article allows us to discuss the scientific theory of gases and therefore the assumptions thought-about for the scientific theory of gases.
Kinetic Theory of Gases
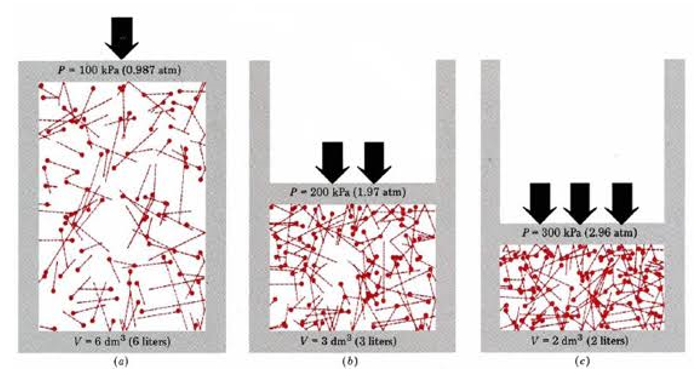
In the 19th century, scientists James Clark Maxwell, Rudolph and Clausius developed the kinetic theory of gases in order to explain the behavior of gases. The theory explains gas as a group of small, hard spheres that interact with each other and with the surface of the wall. The spheres represent the gas molecules and they behave according to the law of motion developed by Newton in the 17th century. It describes how molecules influence gas characteristics such as temperature and pressure. It also explains why gases follow Boyle’s law.
We have learned that the pressure (P), volume (V), and temperature (T) of gases at low temperature follow the equation:
PV = nRTWhere
n = number of moles in the gas
R = gas constant having value 8.314JK−1mol−1
Now, any gas which follows this equation is called ideal gas. Hence the equation is known as the ideal gas equation. But there are certain assumptions that we consider for describing ideal gas behavior.
Assumptions of Kinetic Theory of Gases
All gases are made up of molecules which are constantly and persistently moving in random directions.
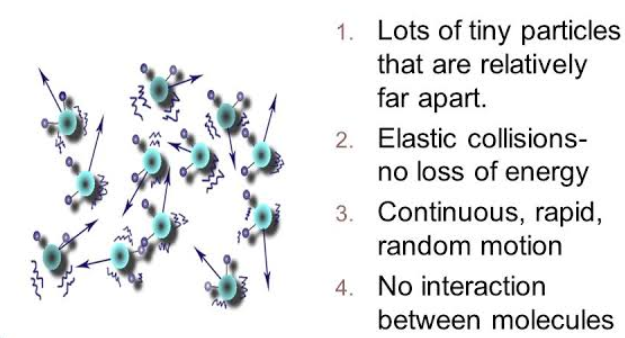
- The separation between the molecules is much greater than the size of molecules.
- When a gas sample is kept in an exceedingly instrumentation, the molecules of the sample do not exert any force on the walls of the container during the collision.
- The time interval of collision between 2 molecules, and between a molecule and the wall is considered to be very small.
- All the collisions between molecules and even between molecules and wall are considered to be elastic.
- All the molecules in a certain gas sample adapt Newton’s laws of motion.
- If a gas sample is left for a sufficient time, it eventually comes to a steady-state. The density of molecules and the distribution of molecules are independent of position, distance and time.