The first law of thermodynamics states that the energy can be converted from one form to another form by the interaction of work, internal energy, and heat. But under any circumstance, there is no creation or destruction of the energy.
Mathematical Expression of First Law of Thermodynamics
Mathematically, this law can be represented as follows.
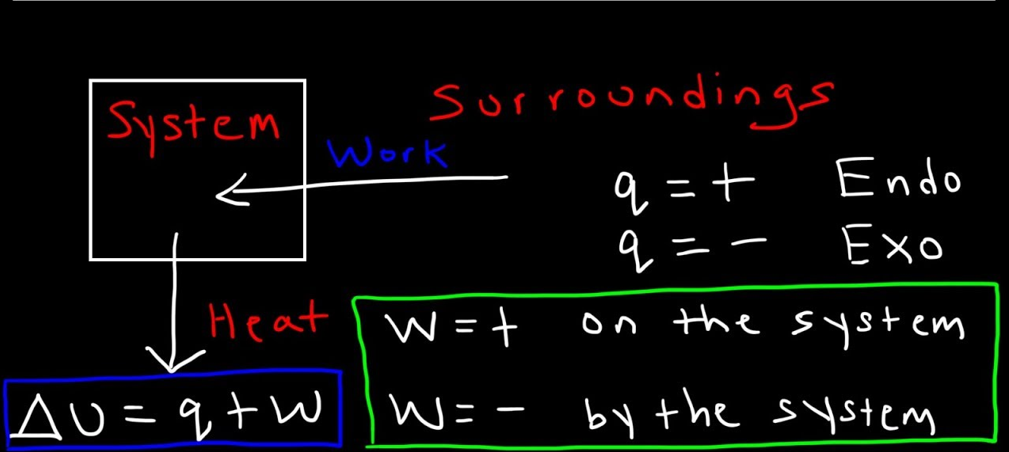
ΔU=q+w
Here ΔU represents the total change in the internal energy of the system. The q is the amount of heat that is exchanged between the system and its surroundings. Whereas, w is the work done by the system or on the system.
According to this law, the internal energy of the system is equal to the work that is being done on that system plus or minus the heat that is flowing in or out the system, so it is just a restatement about the conservation of the energy. The first law of thermodynamics is put into action, by consideration of the flow of energy across the boundary which causes the separation of the system from the surroundings.
Demonstration of First Law of Thermodynamics
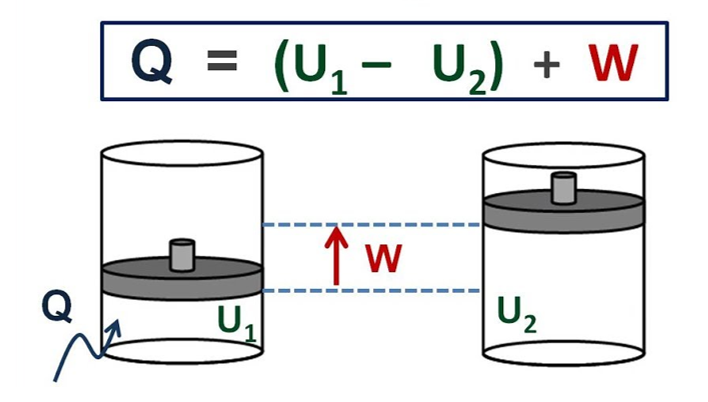
The first law of thermodynamics can be best demonstrated with the example of the gas which is enclosed in the cylinder having the moveable piston. The walls of the cylinder are the boundary and separate the gas inside the cylinder from the outside environment. A mechanism is provided by the moveable piston for the gas for doing work by expanding against the forces that hold the piston. If the work is done by the gas and it expands or either it absorbs the heat from the surroundings by through the walls of the cylinder, then it is corresponding to the net flow of energy across the boundary to its surroundings.
Examples of First Law of Thermodynamics
The transfer and transformations of energy according to the first law of thermodynamics can be observed in our surroundings at all times. Such as the light bulbs convert the electrical energy into radiant energy, When the one pool ball hits another ball, then the transfer of the kinetic energy takes place and which causes the movement of the second ball. The sunlight energy is converted by the plants to the chemical energy which is then stored in the organic molecules. The chemical energy obtained by eating the snack or the food is converted to the kinetic energy by breathing, walking, and by another kind of movement of the body. Additionally, the first law of thermodynamics has various applications in the isolated system, cyclic processes, boiling processes, and melting processes, etc.