p-Block Elements: Group 15
Ammonia and Nitric acid
Ammonia is a colourless gas.It has a high heat of evaporation in its liquid form. Liquid ammonia resembles water in its physical behaviour. It forms strong hydrogen bonds.Its dielectric constant is around 22 at -34°C.Ammonia molecule is shaped in a trigonal pyramidal with the nitrogenatom at the apex. It contains 3 bond pairs and 1 lone pair. N is sp3 hybridised. Liquid ammonia has lower reactivity towards electropositive metals and dissolves many of them. AgI is insoluble in water but soluble in ammonia.
Ammonia is extremely soluble in water.
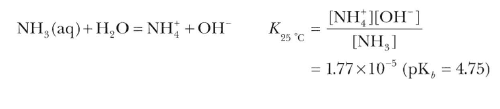
Ammonia is basic in nature and it can form ammonium salts with acids, e.g., NH4Cl, (NH4)2SO4, etc. Being a weak lewisbase, it can precipitate hydroxides of several metals from their salt solutions.
2 FeCl3 + 3 NH4OH → Fe2O3xH2O (brown ppt) + 3 NH4Cl
Cu2+ (aq, blue) + 4 NH3(aq, deep blue) → [Cu(NH3)4]2+(aq)
Ag+ (aq, colourless) Cl‐ → AgCl (s, white ppt)
AgCl (s, white ppt) + 2 NH3 (aq) → [Ag(NH3)2]Cl)(aq,colourless)
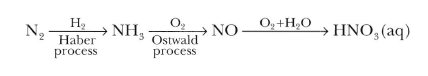
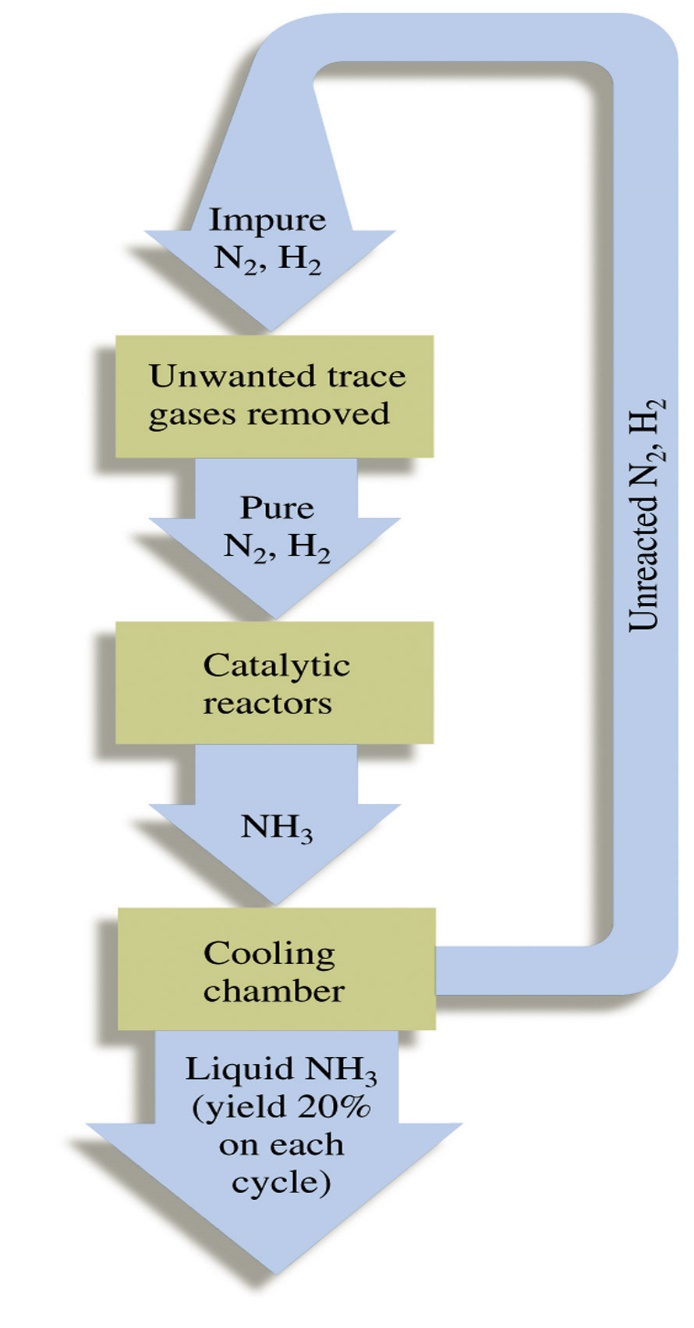
The sequence in industrial utilization of atmospheric nitrogen to form ammonia and nitric acid is:
 |
|
 |
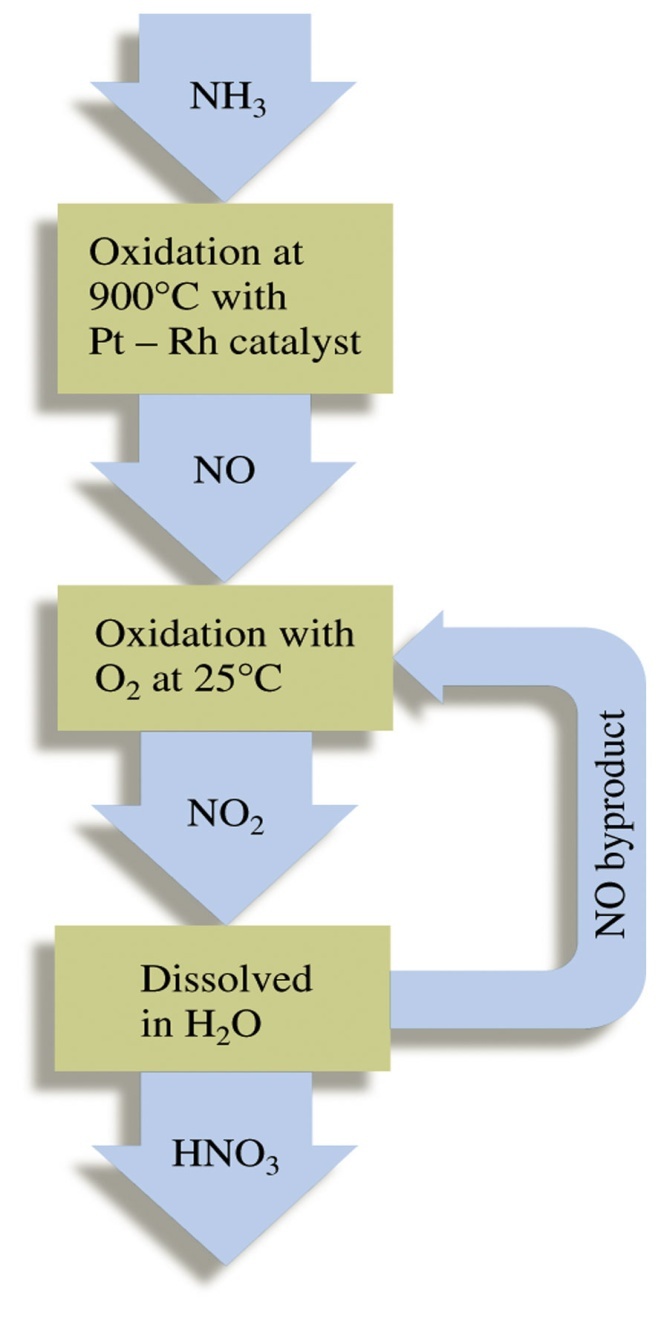 |
| Fig 1a: The Haber process | Fig 1b: The Ostwald process |
The Haber Process is used in the manufacturing of ammonia from nitrogen and hydrogen and then goes on to explain the reasons for the conditions used in the process. This process joins nitrogen from the air with hydrogen obtained from natural gas (methane) to form ammonia. The reaction is reversible and follows Le Chatelier’s principle. The production of ammonia is exothermic.
N2(g)+3H2(g)⇌2NH3(g); with ΔH=−92.4kJ/mol
Conditions required for this process 400-450°C, 200 atm with FeO as a catalyst and small amounts K2O and Al2O3.
Nitric acid, HNO3 is both an acid and an oxidizing agent. At laboratory scale, nitric acid is made by heating KNO3 orNaNO3 and concentrated H2SO4 in a glass retort:
NaNO3 + H2SO4 → NaHSO4+ HNO3
At industrial scale, nitric acid production is done by the catalytic combustion of ammonia. It was developed by Ostwald around the turn of the century and was in production by 1906.
1) Catalytic Combustion:
The catalyst is platinum-rhodium and the reaction occur at 900°C. Rhodium strengthens the catalysts so that its catalytic cycle increase.
4NH3 + 5O2 4NO + 6H2O
2) Oxidation of NO
2NO + O2 2NO2↔ N2O4
3) Absorption
3NO2 + H2O 2HNO3 + NO
NO formed by catalytic combustion is recycled and the aqueous HNO3 can be concentrated by distillation upto ~ 68
Nitric acid is used for making fertilizer and explosives. The nitrate (NO3–) also acts as an oxidizing agent. All nitrate salts are water soluble.
The brown ring test for ensuring the presence of nitrates depends on the ability of Fe2+ to reduce nitrates to nitricoxide, which reacts with Fe2+ to form a brown coloured complex.
KNO3 +H2SO4 KHSO4 +HNO3
6FeSO4 + 3H2SO4 + 2HNO3 3Fe2(SO4)3 + 4H2O +2NO
FeSO4 + NO +5H2O [Fe(H2O)5NO]SO4
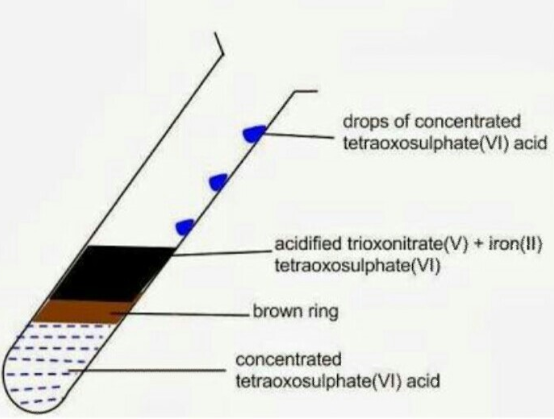
Fig 2: The brown ring test.