Chemical Thermodynamics – Types of systems
A system is referred to as a part of the universe under observation while is the remaining universe constitutes the surroundings with which the system can interact. System and surroundings together make the universe
The system can be classified according to the movement of energy and matter in and out of a system. The matter is easy to understand and includes atoms, ions, electrons, etc. Energy may be transferred to the system as heat, electromagnetic radiation etc. In thermodynamics, the two modes of transfer of energy to the system considered are Heat and Work.
- Heat and work are ways of distribution of energy and not ‘energy’ itself.
- Once within the system, the piece which came via work and the piece which appeared via heat, cannot be prominent
- Before the process is started and after the process is completely done, the terms heat and work are opposite.
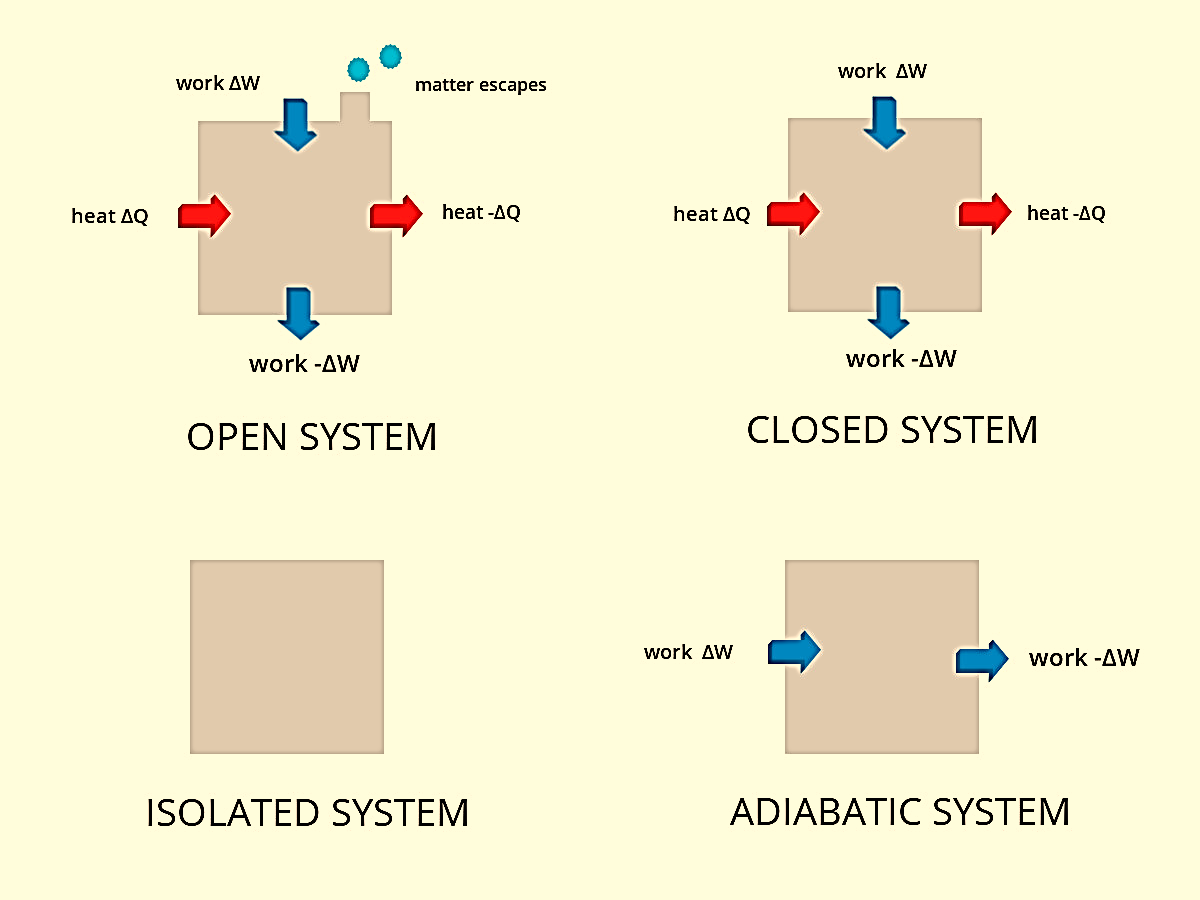
Figure 1: Types of system
In an open system, the exchange of matter and energy takes place between system and surrounding. It is also known as a control volume system. Boundaries of a controlled volume system can be real or imaginary. For example, an open beaker containing reactants is an open system. In this case, the boundary is an imaginary surface that encloses the reactants and the beaker. Another example of an open system is a boiling pot of water without a lid. From the pot heat and steam can both easily escape into the air making mass and energy transfer possible. Most engineering devices are open systems.
In a closed system, no exchange of matter takes place between the system and surrounding but energy exchange is possible. It is also known as a control mass system. For example, reactants present in a closed vessel made up of a conducting material such as copper or steel is a closed system. For instance, water boiling in a pressure cooker is a closed system where the heat can be transferred from the stove to the water as well as the surroundings. In this case, steam has no outlet.
In an isolated system, no exchange of matter or energy can take place between the system and the surrounding. An example of this case is the presence of reactants in a thermos flask or any other insulated vessel which is completely sealed.
In an adiabatic system, heat is not able to cross the boundary. A coffee mug and its immediate surroundings make up for an adiabatic system with an imaginary boundary. An isothermal system is one where the temperature remains constant. An isobaric system is one where the pressure remains constant while in an isochoric system volume remains constant.
Table 1: Types of system
| Types of System | Mass Transfer | Energy Transfer | Examples |
| Open | Yes | Yes | Pump, Compressor, Piston Cylinder with valves |
| Closed | No | Yes | Piston-cylinder without valves |
| Isolated | No | No | The universe, hot coffee in a perfectly insulated thermos, gas balloon |
| Adiabatic system | Yes | No | Turbine |
A system can be considered homogeneous when all the constituents present in the same phase and are uniform throughout the system. A mixture of two miscible liquid is a good example. A system is considered heterogeneous when it consists of two or more phases where the composition is not uniform. A mixture of insoluble solid in water is a good example.