Alkynes –nomenclature
Alkynes are unsaturated hydrocarbons with the triple bond(-C). They are represented by the general formula: . Alkynes are named in the same way as alkenes by replacing suffix ane of alkane by yne. In higher members, the position of triple bond is indicated by giving numbers 1,2,3,4,…etc. to the carbon atom in the molecule. The numbering of the chain is always done from one end in such a manner that the triple bonded carbon atom gets the least number. For example,
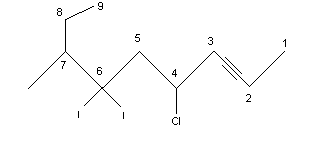
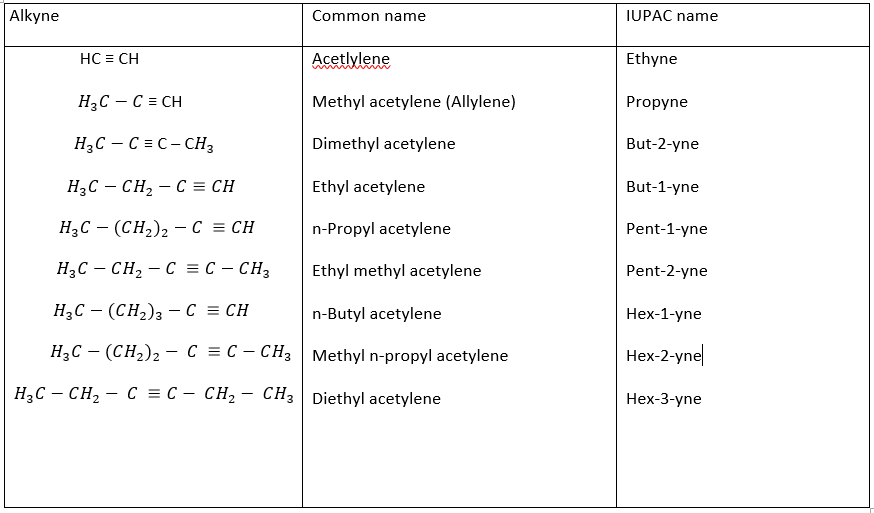
The IUPAC and the common name of a few important members of the family are given below:
Now let us discuss rules for the nomenclature of organic compounds:
Rule 1: longest chain rule: select the longest continuous chain of carbon that includes triple bond. The selected chain, containing the maximum number of carbon atoms, is regarded as a parent chain or root chain and it gives the name of the parent hydrocarbon. The remaining carbon atoms, which are not included in the parent chain are termed as substituents or branched chains.
Rule 2: the position of the triple bond: number the carbon atom of the parent chain as 1,2,3… etc. starting from the end which gives a lower number to the carbon-carbon triple bond.
Rule 3: naming the substituents or the lowest set of locants rule: when two or more substituents are present, then the end of the parent chain which gives the lowest set of the locants is preferred for numbering. This rule is called the lowest set of locants. This means that when two or more differently set of locant are possible than the set of locants which when compared term by term with other sets has the lowest term at the first point of difference each in the order of increasing magnitude. This lowest set of locants rule is used irrespective of the nature of the substituents. If the same substituent or side chain occurs more than once, the prefix di, tri, tetra, penta, hexa… etc., are attached to the names of the substituents. It may be noted that the positions of the substituents are indicated separately and the numerals representing their positions are separated by commas.
Rule 4: naming different substituents present in the parent chain: if the parent chain has two or more different substituents are present in the molecule, they are named in the alphabetical order along with their appropriate positions. It must be noted while naming that the prefixes di, tri, etc. are ignored while comparing the substituents.
When there are two triple bonds present in the molecule, find the longest carbon chain including both the triple bond and number the chain starting at the end closest to the triple bond that appears first. The suffix, in this case, would be –diyne.
Rule 5: naming different substituents at equivalent positions: if two different substituents are in equivalent positions from the two ends of the chain, then the numbering of the chain is done in such a way that the group which comes first in the alphabetical order gets the lowest number.
Rule 6: the molecules containing a triple bond are called alkynyl.
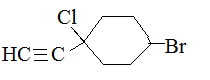 1-chloro-1-ethynyl-4-bromocyclohexane
1-chloro-1-ethynyl-4-bromocyclohexane