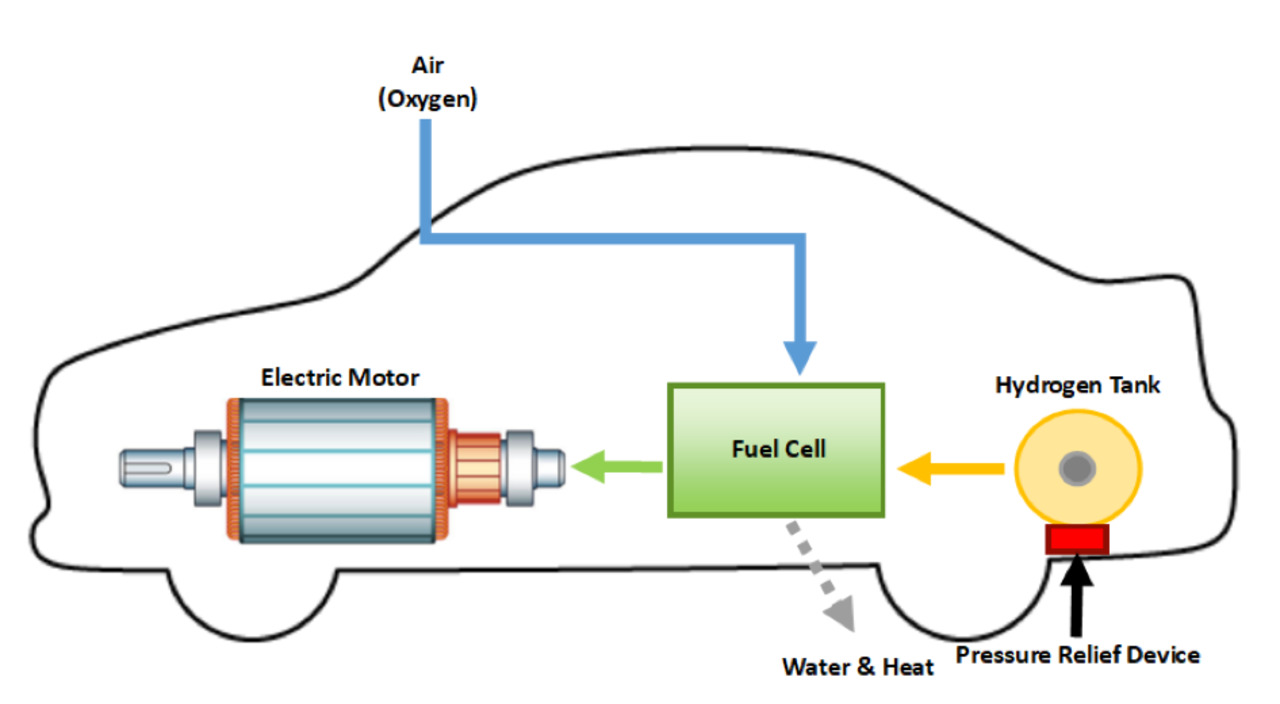
Fuels burn to release a large amount of energy. The energy is released by the combustion of some fuels such as dihydrogen, methane, LPG, and gasoline. Here we are going to talk about hydrogen as a fuel. Dihydrogen releases about three times more energy than gasoline on combustion. Also, the pollutants in the combustion of dihydrogen are very less. The only pollutant will be dinitrogen, which is because of the impurity of dihydrogen. This can be easily reduced by injecting a small amount of water into the cylinder to lower the temperature so that the dinitrogen and dioxygen won’t be able to react with each other. Liquid hydrogen is used as an important rocket fuel. And as we know the primary condition for any rocket fuel is that its mass should as small as possible for a given amount of energy produced. Hydrogen has low mass and its enthalpy of combustion is high hence, it is considered as ideal rocket fuel. Hydrogen as a fuel was first used in Saturn-V rocket that enabled first astronauts to land on the moon and since then it has been the main fuel in the space shuttle rockets. Both hydrogen and oxygen which needed to burn the hydrogen are carried on the rocket in the liquid form.
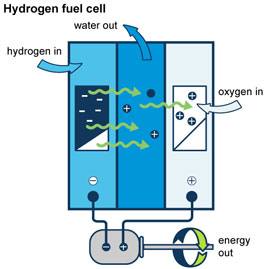
Now let us look at the advantages and disadvantages of hydrogen as a fuel.
Advantages of hydrogen as a fuel:
- Hydrogen releases a greater amount of energy per unit weight of the fuel.
- On combustion of hydrogen, there is no emission of environmental pollutants such as CO, CS, oxides of nitrogen(NO), aldehydes, hydrocarbons, and lead compounds, etc.
- The main advantage is that only product of the combustion of hydrogen is water.
- For use of hydrogen as a fuel, internal combustion engines can be easily modified.
- Hydrogen Fuel cells for generation of electric power with the conversion efficiency of 70-85
Disadvantages of hydrogen as a fuel:
- Hydrogen is highly combustible and because of this nature, it burns with the explosion. Hence, it is difficult to handle safely.
- Hydrogen is not easily stored or transported from one place to another. A cylinder of compressed hydrogen weighs about 3o times as much as a tank of petrol containing the same amount of energy. Also, hydrogen is converted into a liquid state by cooling to 20K and this would require expensive insulated tanks.
- It is an expensive fuel because the cost of production is high.
Moreover, Hydrogen is abundant in the form of water in the oceans that could meet all of our energy needs and can provide inexhaustible energy supply. But the main problem is to extract it from water. This is because water decomposes into hydrogen and oxygen as shown below:
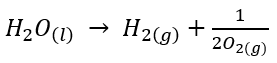
This decomposition requires 286kJ energy per mole of water. Therefore, the reaction is difficult to carry out economically. Thus, because of a large amount of electricity is required, electrolysis becomes an expensive method and can be carried out only when electricity is cheap. In 2005, India used dihydrogen as a fuel in automobiles, under the pilot project. Initially, about 5