Chemical Thermodynamics: Second law of thermodynamics
The second law of thermodynamics is derived from three statements.
Carnot’s Theorem states that “the efficiency of an engine working irreversibly is always less than a reversible engine operating between the same two reservoirs while the efficiency of two reversible engines is the same ”.Carnot’s engine is the highest possible heat engine using hot and cold reservoirs.
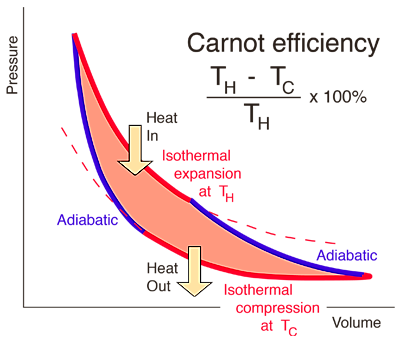
Figure : Carnot cycle
However, it is impossible to build a cyclic machine that converts heat into work with 100
Some heat energy is required to for the random molecular motion and mechanical work, which is ordered motion. Hence, the unavailable work is due to the role of Entropy in the process.
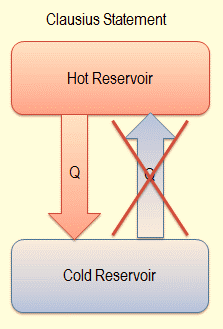
Figure : Clausius statement
Clausius’s statement of the second law states that “Heat does not ‘flow’ from a colder body to a hotter body, without a concomitant change outside of the two bodies. This concludes that the spontaneous direction of the flow of heat is from a hotter body to a colder body.To make heat flow from a cold body to a hot body, there must be accompanying change elsewhere which means that the work has to be done to achieve this.
The Kelvin’s and Clausius’s statements of the second law are equal if we see that clearly. If we say Kelvin’s statement is not appropriate, then the Clausius’s statement will be appropriate as well of the second law (and vice-versa).
Figure : The second law of thermodynamics. The combined system of the heat engine and pump act as a heat engine with a single reservoir which is the violation of Kelvin-Planck statement. Hence, net heat loss in a system is certain.
| HEAT ENGINE | HEAT PUMP |
| a heat engine receives heat from a high-temperature reservoir or SOURCE | A heat pump(HP) or refrigerator (R) receives heat from a low-temperature medium (icebox of the refrigerator, outdoor air, etc. |
| Heat is converted to work and remaining heat is rejected to a low-temperature SINK. | Work is done to remove heat from the SOURCE. In the refrigerator, low temperature is maintained while in heat pump the heat is absorbed from cold SOURCE and supplied to the warmer SINK |
| QNET = WNET
Efficiency= η |
A thermal reservoir is a body that can provide or absorb heat without changing its temperature.High-temperature reservoirs is a source while the low-temperature reservoir is a sink. They do not change in temperature against entropy change.
Entropy is a term defined to explain the degree of randomness or disorder in a system. The entropy of a closed system increases during any spontaneous change/process. If we consider the Universe to be a closed system, then the entropy of the universe will increase during any spontaneous change. And, all thermodynamic processes must satisfy both the 1st and 2nd Laws.
Change is entropy is defined as:
∆S = dq/T
Where, dq is the change in heat associated with the system and absolute temperature, T.
∆Sisolated> 0 (a statement of entropy non-conservation)
ΔSuniverse=ΔSsystem+ΔSsurroundings≥0
Hence, in an ideal reversible process or an isentropic process, the overall entropy of the system remains constant.