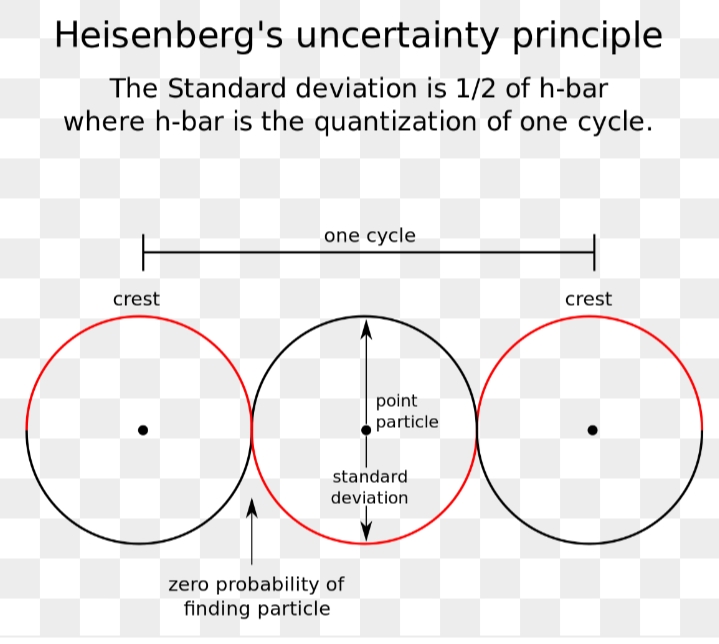
Heisenberg uncertainty principle is also known as indeterminacy principle. It is a part of quantum mechanics. It is segmented in year 1927 by a German physicist Werner Heisenberg. It states that at the same time the position and the velocity of an object cannot be measured together exactly even theoretically. It took people time to accept it because it’s contrary to our real life experience where we can independently measure position as well as motion. There is no clue of this principle by any ordination experiments. For example the momentum and position of an electron cannot be calculated exactly. Practically it means the orbitals occupied by electrons cannot be calculated with high correctness. The concept of measuring it exactly is illogical.
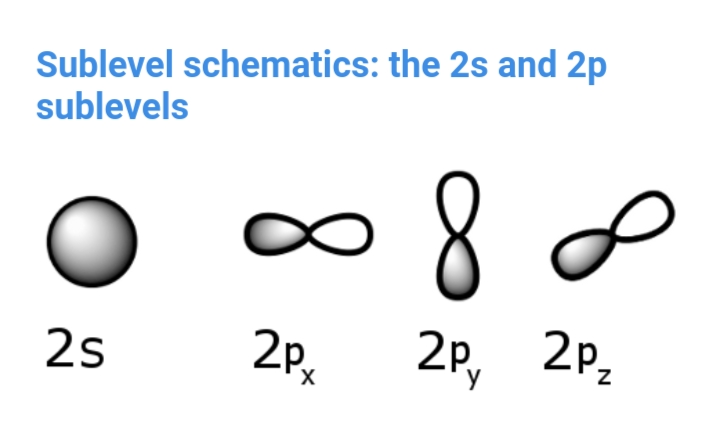
Orbitals are based on probability function for an electron. Such as and orbital is drawn in a way that most probably at a given time the electron will be within its volume. It is notable only for very small objects (atomic or subatomic particles)
Typical Orbitals:
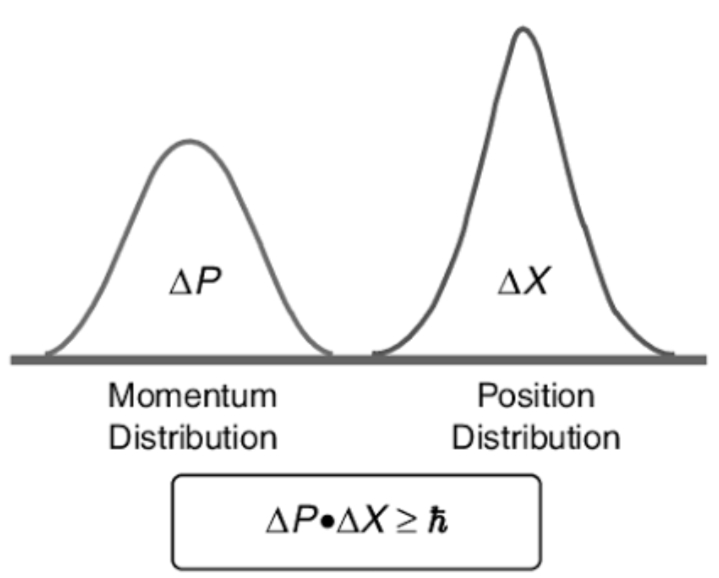
This principle specifies that the product of the uncertainties in position and velocity is equal to or greater than a small physical quantity (h/(4π)
Where:
h = Planck’s constant (6.6 × 10−34 joule-second).
For only the extremely small masses of atoms and subatomic particles the product of the uncertainties in position and velocity is important.
It is not easy to understand this principle but it’s not even that difficult. Assume that you are trying to examine an electron through a microscope in a laboratory. You want to measure its position and velocity. The light that you are using in this observation reflects from the electron and come to your eyes. Only this way you can see it. But the light affects the electron when it reflects back.
Light contains tiny particles known as photons. These particles have momentum. The amount of momentum of light depends on the wavelength (color) of the light waves. It can be controlled in a laboratory.
If the light you use for our observation has photons with a lot of momentum, then you could easily see the position of electrons such as if shin a really bright light into the microscope, they will have a lot of momentum. They’ll transfer it to the electron when they reflect it and speed it up. This will make it hard to calculate its speed. Our observation has affected the electron’s velocity.
But if the light we use has photons with least momentum, we can’t easily see the electron’s position. But since the photons have less momentum, they won’t affect the electron’s speed and make it easier to know how fast it is moving i.e. its velocity.
The more accurately you know the motion of an object, the less accurately you can know its position. This is the Heisenberg uncertainty principle.
Heisenberg and Quantum Mechanics:
The quantum mechanics is all about exploring the subatomic particles. In quantum world things behave in quite a different manner than they do in reality. The foundations of quantum mechanics was made by Einstein and Planck in the 20th century. Ideas were turned into equations and laws.