General Principles and Processes of Isolation of Elements:
Copper Extraction
Copper is a reddish orange bright metallic substance that is an excellent conductor of heat and electricity. It can be drawn into pipes and it is resistant to corrosion.
The most common found copper ore in nature is chalcopyrite which is a mixed ore of CuFeS2. It contains only 0.5

Fig 1: Ores of copper
Concentration
TheFroth-Floatation process is used to concentrate the finely crushed copper ore obtained from mining. The ore is suspended in water with a small amount of pine oil. A blast of air is passed through the suspension. The particles get wetted by the oil and float as a froth which is skimmed.The gangue sinks to the bottom. Flotation of the ore discardssome amount of iron and increases the mass
Roasting
Roasting oxidizes the FeS to FeO but leaves the CuS unreacted.
2CuFeS2 + O2 Cu2S + 2FeS + SO2
S + O2SO2
4As + 3O2 2As2O3
4Sb + 3O2 2Sb2O3
Cuprous sulphide and ferrous sulphide are further oxidized into their oxides.
2Cu2S + 3O2 2Cu2O + 2SO2
2FeS + 3O2 2FeO + 2SO2
Smelting
The roasted ore is mixed with coke and silica (sand) SiO2 and is introduced into a blast furnace. The hot air is blasted and FeO is converted into ferrous silicate FeSiO3. Heating in sand converts FeO to FeSiO3, a molten slag.
FeO(s) + SiO2(g) → FeSiO3(l)
Cu2O +FeS → Cu2S +FeO at this high temperature.
Bessemerization
Copper metal is extracted from molten matte by a process called bessemerization. The matte is introduced into Bessemer converter which upholds by tuyers. The air is blown through the molten matte. A blast of hot air converts Cu2S into Cu2O which then reacts with remaining Cu2S to give molten copper with some impurity in the mixture.
2Cu2S + 3O2 2Cu2O + 2SO2
2Cu2O + Cu2S 6Cu + SO2
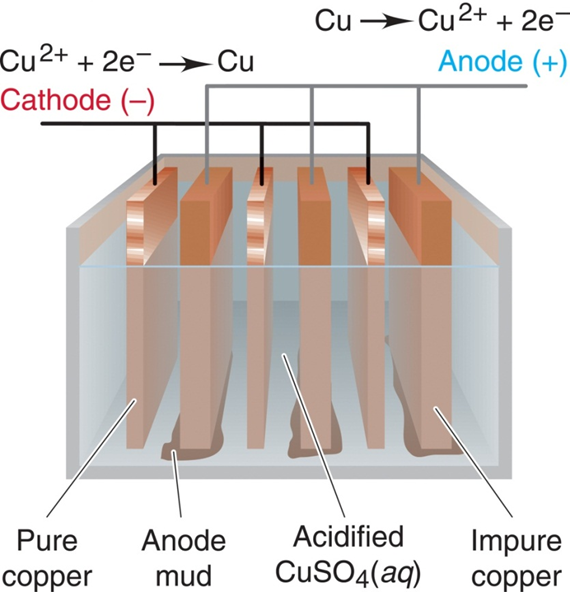
Refining or Electrowinning
Blistercopper is refined by electrolysis. Blocks of blister copper obtained from bessemerization are used as anodes in electrolysis while thin sheets of pure copper act as cathodes. The cathode plates are coated with a lining of graphite to remove depositing pure copper. The electrolyte is copper sulphate (CuSO4) mixed with a little amount of H2SO4 to increase the electrical conductivity. Optimum potential difference required for this process is 1.3 volt. During electrolysis, pure copper is deposited on the cathode plates and impurities which are soluble and fall to the bottom of the cell as anode mud or sludge.
At the negative electrode:
Cu2+ + 2e– Cu (reduction)
At the positive electrode:
Cu Cu2+ + 2e– (oxidation)
Copper is an excellent conductor and does not corrode quickly which makes it a good material for wiring and plumbing. Copper for the wiring needs to be 99.99