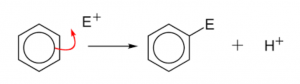
Aromatic hydrocarbons – mechanism of electrophilic substitution reaction
The orbital structure of the benzene makes it quite clear that the π-electron cloud lying above and below the plane of the benzene ring is held loosely and is thus, available to the electron seeking reagents i.e., electrophiles. Therefore, the substitution reaction in benzene is electrophilic in nature. These reactions are characteristic not only of benzene ring but also of the compounds where benzene ring is present. The electrophilic substitution reactions proceed via the following three steps:
- Generation of the electrophile
- Formation of a carbocation intermediate
- Removal of a proton from the carbocation intermediate to form the product.
Step1. Generation of the electrophile:
The attacking reagent may not be a strong electrophile, therefore, first of all, an electrophile is generated by some preliminary reaction.
For example, during the chlorination of benzene, an electrophile is generated by reacting with anhydrous used as the catalyst. Similarly, during the alkylation of benzene, electrophile is generated as:
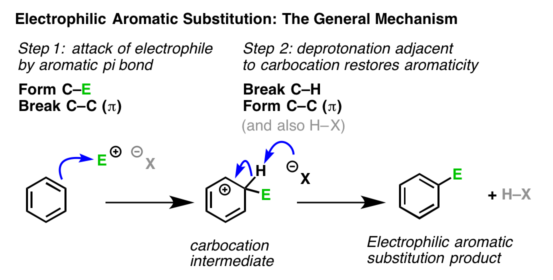
Step2. Formation of carbocation intermediate:
The electrophile attacks the π-electron cloud of the aromatic ring and forms a bond with carbon, creating a positive charge on the ring. This results in the formation of a sigma complex or a carbocation.
The arenium ion gets stabilized by resonance
The resulting carbocation has three important contributing structures that spread the positive charge over the remaining carbon atoms, although more on the carbon atoms that are ortho and para to the position attacked by the electrophile.
It may be noted that during the formation of the sigma complex, the aromatic character of the benzene ring is lost. This is because in the carbocation, one of the carbon is hybridized and delocalization of electrons stops at
hybridized carbon. Therefore this step is slow and hence is the rate-determining step of the reaction.
Step3. Removal of a proton:
The carbocation formed loses a proton to the nucleophile present in the reaction mixture to form a substitution product. The aromatic character of the benzene ring is restored during this step and this step is fast. The two electrons from the carbon-hydrogen bond move to regenerate the aromatic ring by the loss of proton and thus restoring the aromatic character.
Thus, the electrophilic substitution in benzene involves addition followed by elimination and results in the desired substituted product.
In almost all the electrophile aromatic substitution reactions of benzene, a catalyst is generally required to generate a powerful electrophile i.e., the attacking reagent. The catalysts are usually Lewis acid or protonic acids. For example, chlorination of benzene requires the presence of a Lewis acid catalyst, usually the ferric chloride. Ferric chloride being electron deficient in nature takes up ion from chlorine molecule to make it, polar and to generate electrophile i.e., chloronium ion as follows:
In the alkylation and acylation reactions of benzene, the catalyst employed is anhydrous aluminium chloride which is also a Lewis acid. Similarly, sulphuric acid acts as a catalyst in the nitration and sulphonation. It is interesting to note that no catalyst is required in the electrophilic addition reactions of alkenes. In fact, there is no delocalization of the π-electron charge in alkenes. The π-electron cloud can easily cause the polarization of the attacking molecule to generate the electrophile. However, in benzene and other aromatic compounds, there is delocalization of electron charge. The electron clouds are incapable of causing any polarization of the attacking reagent. Here, a catalyst i.e., Lewis acid or protonic acid is always required to generate the electrophile.