The lead accumulator refers to a secondary cell as the electrical energy doesn’t generate itself inside the cell, but it is stored prior to an external source.
It’s a reversible cell as the cell reactions are in the reversed state if external emf is suitably bigger than this cell’s emf which is applied. Therefore, in this cell, total cell reaction are reversed by exerting external opposite to e.m.f which is greater than e.m.f of the cell.
This very cell has the capability to store the electrical energy from charging or from the external source, and can supply process is done at the time of discharge. In chemical energy form, the energy hence is stored. So, this cell refers to an accumulator or the storage cell or the storage battery.
Cells’ voltage is not dependent on electrodes’ size or its cell’s size, but it’s definitely dependent on the strength of the solution of sulphuric acid.
Construction:
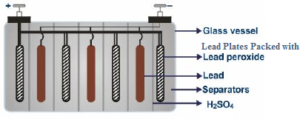
The lead accumulator is having lead plates in the form of a negative electrode. The lead plates are brimmed with lead oxide and behave as a positive electrode. The positive and negative electrodes are organized in an alternate way.
This accumulation of the lead plates are immersed in a vessel, non-conducting in nature and made out of plastic or glass or ebonite which are non-conductors of electricity and contains 38
The whole of the positive plates are associated with each other plus the entire negative plates are connected amongst each other.
Representation of Cell:
Working:
Cell Discharging: When the cell starts to work, oxidation occurs on the lead plates and the reduction occurs in the lead plates with PbO i.e. Lead Oxide. This is known as the discharging of the cell.
The Reaction at the negative electrode (Anode)
The reaction at the positive electrode (cathode)
Now, the net reaction of the cell during its discharging
Therefore, at the time of discharging, sulphuric acid gets converted into H2O (water) and thus, Sulphuric acid’s specific gravity gets reduced to “1.17”
Charging of cell: When emf acting externally is greater than cell’s emf which is applied, then there are accurate reverse reactions taking place. The Oxidation occurs on positive electrodes and reduction at negative electrodes. This is referred to as the charging of the cell.
Lead Accumulator Uses:
Lead accumulators have their use in motor cars and in different automobiles vehicles as a consequence, they are well-known as “car batteries”. Generally, six cells are attached in series to offer the voltage of 12V.
- Lead accumulators are used in telegraph offices and telephone.
- They have the use of radio sets, electric clocks, burglar’s alarms, etc.
- They have their usage in scientific laboratories as the constant DC voltage source.