Chemical Kinetics: Concept of collision theory
If molecules in a chemical reaction react at different rates there must be specific conditions that play a role in determining the rate.The rates of reaction are associated with the particles and their properties through a model called collision theory. Collision theory explains how changing reaction conditions control reaction rates.Particles undergo reaction only when there is a successful and effective collision between them.For instance, gas molecules travel with different velocities and have different kinetic energies. The average kinetic energy is dependent on temperature. Molecules with KE are capable of movement which then results in a collision. Therefore, three things that must happen before a chemical reaction will take place.
- Molecules must collide
Fig : This is 2 body collision where two reactant molecules collide to produce products.
- During the collision, the Molecules Must be in the Proper orientation. When two molecules react, bonds need to be broken and bonds need to be created. For this to happen molecules need to be in the correct orientation when a collision occurs.They must collide in the acceptable orientation to produce an activated complex. The activated complex is a temporary, unstable arrangement of atoms that may lead to products. It can be thought of as a transition state between reactants and products.For example, at room temperature and pressure N2 and O2 molecules undergo approx. 1010 collisions/sec. Yet the reaction time is long because not all collisions lead to a reaction. Only a minute fraction of all the collisions lead to a net change in reaction.
Fig : Reaction takes place at a specific orientation of the molecules. No reaction occurs with poor orientation.
- The reactants must have sufficient kinetic energy when they collide for the reaction to occur. In other words, they must collide with sufficient velocity and a specific minimum amount of energy (activation energy) to form the activated complex. The high activation energy of a reaction indicates that only a few collisions have the required energy and the reaction rate is slow.Most reactions, even exothermic reactions, require energy to occur.
Fig : Collision also requires sufficient velocity. Even though the orientation may be right, without enough KE there is insufficient energy to break bonds that need to be broken before new bonds can form
The rate of a reaction can be increased by manipulation some factors in a reaction, such as a temperature change or concentration change of reactants. These factors increase the reaction rate by producing a greater number of effective collisions.
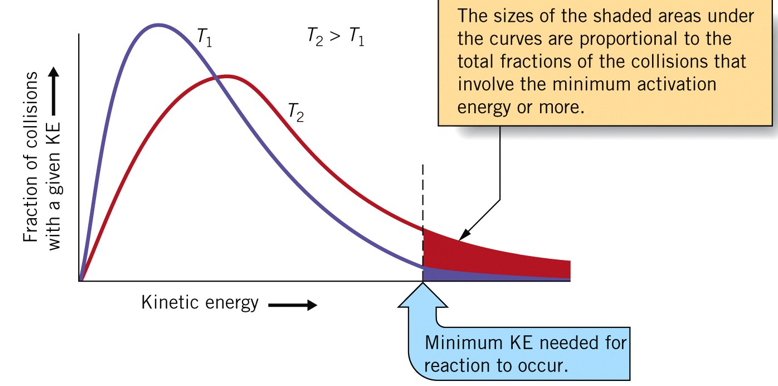
Fig : As temperature increases, more molecules gain activation energy, Ea and then undergo a reaction to form products.
Likewise, a greater number of effective collisions can be achieved by anything that
- Increases the energy of the reactants
- By increasing the temperature
- Lowers the activation energy
- By using a catalyst
- Produces more collisions per unit of time
- By increasing the concentration of the reactants
- By increasing the pressure of the reactants
- By increasing the surface area per volume ratio of the reactor