Both conductivity and molar conductivity depends upon the electrolyte’s concentration. The Molar Conductivity and Conductivity of both strong and weak electrolytes decreases as there’s decrement in concentration as the ions’ number per unit volume which carries the current in the solution goes down on dilution.
Solution’s Conductivity at a specific concentration = Solution’s Conductance kept between 2 electrodes of platinum
where,
Volume of solution = 1 unit
Cross-section area of electrodes = 1unit
Distance = 1 unit
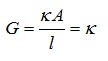
Molar conductivity of a solution at a specific concentration = Conductance of solution where
V = Volume of solution
Electrolyte’s Concentration = 1 mole
Electrodes’ Cross-section area = A
Distance = 1 unit
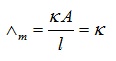
When concentration reaches zero, the molar conductivity is described as the “limiting molar conductivity”. It is given by Ëom symbol.
![]() of electrolytes rises up along with the dilution.
of electrolytes rises up along with the dilution.
The variation observed is contrasting for both weak and strong electrolytes.
For Strong Electrolytes
Given as per the equation:
![]()
![]() refers to the molar conductance at the given temperature plus the molar concentration at infinite dilution respectively.
refers to the molar conductance at the given temperature plus the molar concentration at infinite dilution respectively.
In the above equation, b is constant, which depends on the solvent’s viscosity.
From the graph, you can see that ![]() goes down as the concentration goes downwards.
goes down as the concentration goes downwards.
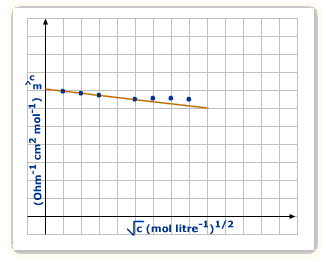
This is the reason at high concentration, there’s bigger Inter-ionic attraction that hinders ions’ movement as the conductance declines ![]() is that very conductance present at infinite dilution. It’s the dilution at what place the ions are at a far distance plus there’s none of the Inter-ionic attraction. This can be acquired by graph’s extrapolation to ‘0’ concentration. Molar conductivity slowly increases with dilution.
is that very conductance present at infinite dilution. It’s the dilution at what place the ions are at a far distance plus there’s none of the Inter-ionic attraction. This can be acquired by graph’s extrapolation to ‘0’ concentration. Molar conductivity slowly increases with dilution.
Weak electrolytes
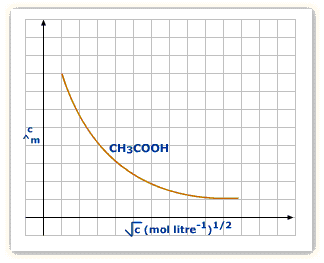
The weak electrolyte separates or dissociates to lower extent enough, therefore, it conductance is lesser as compared to strong electrolyte at the very same concentration level.
A pretty big increment at the infinite dilution is due to the increase in ionization and so as the ions’ number in the solution accelerates.
The value of ![]() can’t be achieved by extrapolation as it can be viewed on the above-mentioned graph. It is attained by implementing Kohlrausch’s law.
can’t be achieved by extrapolation as it can be viewed on the above-mentioned graph. It is attained by implementing Kohlrausch’s law.
![]() worth for strong electrolytes is greater in comparison to weak electrolytes for same amount of concentration. The increase in
worth for strong electrolytes is greater in comparison to weak electrolytes for same amount of concentration. The increase in ![]()
![]() for strong electrolytes is very small comparatively to weak electrolytes.
for strong electrolytes is very small comparatively to weak electrolytes.
For electrolytes, weak in nature, the graph doesn’t represent a straight line; the graph which is drawn between concentration and molar conductivity. The graph represents the reason why weak electrolytes consist of reduced molar conductivities plus reduced dissociation degree at high concentrations that increases abruptly at a lower concentration. Therefore, the use of Kohlrausch’s law is there which is not dependent on ion’s migration to determine to restrict the molar conductivity, Ëm° of weak electrolytes.