Have you smelled freshly-baked bread? Well, most of the people who have who had an aroma of this baking, loved it, and people also enjoy its taste. It’s fluffy because of the yeast on sugar, and also it’s light. The yeast has the ability to convert sugar into carbon dioxide, which causes the dough to swell at high temperature. In the end, it’s pretty pleasant, particularly when covered with butter which is melted.
Charles’s Law
Charles’s Law is defined as it’s the volume of a given mass of gas which changes directly with absolute T of the gas as pressure is unchanged. The absolute temperature, also called, the thermodynamic temperature is measured in Kelvins.
The Kelvin scale should in use as zero showed on the Kelvin scale have a close similarity to an entire blockage of molecular motion.
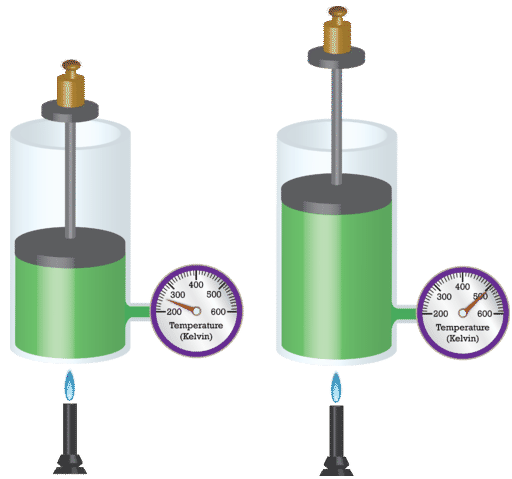
Fig.1
The container having enclosed gas is put on flame and is heated, so the kinetic energy of the molecules increases and it pushes the piston upwards and more technically outwards, as a result of which there’s an increase in the volume.
So, the direct relationship of Charles’s law can be mathematically represented by the below-mentioned equation:
V/T = k
Also, in Boyle’s Law, the K is constant only for a given mass of a gas sample. In the given table, you’ll see the volume and temperature data for a different amount of gas at constant pressure.
The 3rd column is constant for this particular set of data and is equal to the V divided by the Kelvin T, always.
| Temperature | Volume | Constant k = V/T |
| 50 | 20 | 0.40 |
| 100 | 40 | 0.40 |
| 150 | 60 | 0.40 |
| 200 | 80 | 0.40 |
| 300 | 120 | 0.40 |
| 500 | 200 | 0.40 |
| 1000 | 400 | 0.40 |
When this data is graphed, the result is a straight line, indicative of a direct relationship, shown in the figure below.
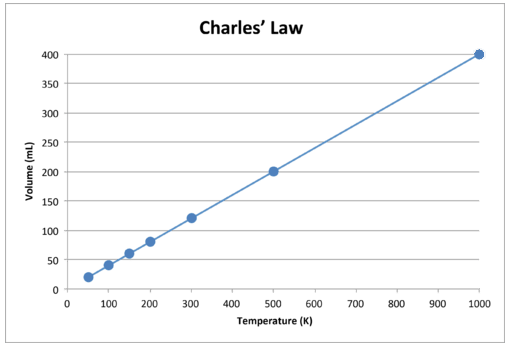
Figure 2: Volume of the gas accelerates as the Kelvin temperature (T) moves up.
Note that the line moves straight to the origin, which means that as the absolute temperature of the gas reaches zero, its volume reaches zero. Nonetheless, when the gas is brought to intensely cold temperatures, its molecules will ultimately reach the liquid state before reaching absolute zero. The temperature at which it turns into a liquid state takes place, changes for different gases.
Charles’s Law can even be used for comparing the changing conditions of the gas. V1 and T1 are the initial volume and temperature of the gas, whereas, V2 and T2 are the final volume and temperature of the gas. And the mathematical representation of Charles’ law is:
V1/T1 = V2/T2, or
V2/V1 = T2/T1, or
V1T2 = V2T1
Temperatures in Celsius will not work. Recall the relationship that
This equation can be used for the calculation of any 1 out of 4 quantities, but on the condition, if other three are known. The direct relationship will be satisfied only if the temperature is expressed in Kelvin. The temperature in Celsius doesn’t work. Do you remember that relationship where,
K= Degree Celsius + 273
This above equation takes place to express temperature in Kelvin.