Chemical Thermodynamics: Internal energy and Enthalpy
The total energy inside a system may change by two formats: either heat and/or work. However, some energy is already present within a system before transformation takes place. The internal energy of a system is defined as the sum of the kinetic and potential energies of the particles that form the system. The term internal energy is used for the total energy inside a system, and the symbol U. In this case, Q and W stand for heat and work, respectively.
Accordingly, energy conservation dictates the First “Law” of Thermodynamics: .
Therefore, the internal energy of an ideal gas, for example, is directly proportional to the temperature of the gas.
This analogy can be defined by:
Usys = 3/2 RT
Here, R is the ideal gas constant in joules per mole kelvin (J/mol-K) and T is the temperature with SI unit ofKelvin.
Enthalpy is a state function and an extensive property, because U, P and V are a state function. In isobaric processes, the energy received by a system due to heat equals to the change in enthalpy.
Accordingly, the change in enthalpy at constant pressure is now given by
ΔH=ΔU+pΔV
Where, U=internal energy, p=pressure and V=volume. However, it is impossible to directly measure the total enthalpy of a system, so we can only measure changes in enthalpy.
A change in enthalpy is the heat produced or absorbed at constant pressure in a specific reaction/process.
Likewise, Several thermodynamic processes can be illustrated as:
Isochoric: Q = ∆U = Qp
Isobaric: Qv = ∆H, in both cases, Q does not depend on the path of work.
Table 1: Difference between Enthalpy and Internal Energy
| Enthalpy | Internal Energy |
| Enthalpy is the heat energy that is being evolved or absorbed during the progression of a chemical reaction | The internal energy of a system is a sum of potential energy and kinetic energy of the system |
| Calculated as H = U +PV | Calculated as |
| Defined as the relationship between the system and the surrounding | Defined as the total energy in a system |
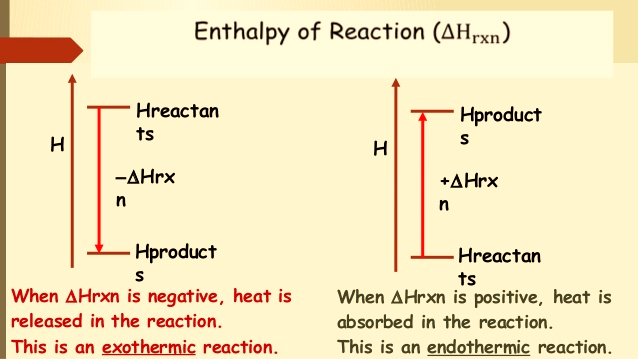
A reaction that absorbs heat has positive “Hr” and is known as an endothermic reaction, whereas a reaction that liberates heat is characterized by negative “Hr” and is known as an exothermic reaction.
| Enthalpy of Reaction ∆Hrxn | |
 |
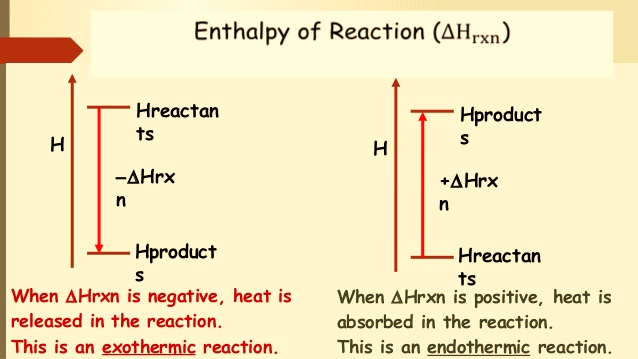 |
| Exothermic reaction | Endothermic reaction |
Enthalpy of formation of a phase is the amount of heat added or released (usually released) during the formation of that phase from its elements at some given T & P. Because heat is a form of energy, Hf can, therefore, be thought of as the “heat content” of that phase. Enthalpy could also be measured for a reaction or a system. It is expressed in joules/mole or calories/mole. The enthalpy of a reaction is therefore defined as the heat released or absorbed during, or as a result of, this reaction.
In a chemical reaction, the enthalpy of reaction (ΔHrxn) is the difference in enthalpy between products and reactants; the units of ΔHrxn are kilojoules per mole. Likewise, reversing a chemical reaction reverses the sign of ΔHrxn.
Standard conditions are defined when 1 mol of a substance is undergoing reaction where the pressure equals 100 kPa and temperature at 298 Kelvin or 25 degrees Celsius.
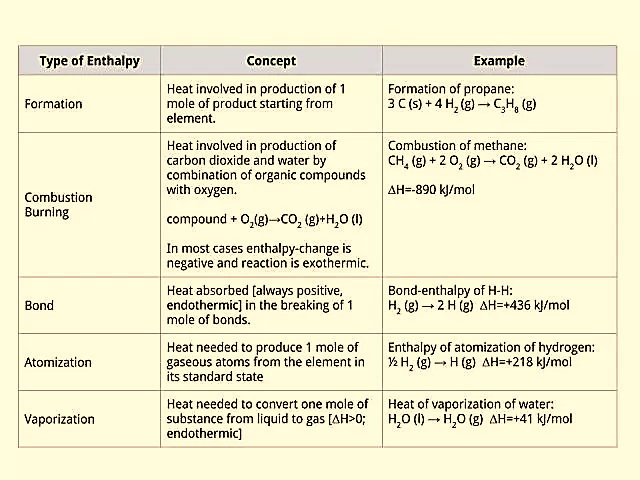
Figure 1: Types of Enthalpy change