Concept of shells and subshells
Shells
An electronic shell or a principal energy level is an orbit followed by electrons around an atom’s nucleus. The closest shell to the nucleus is called the K shell (also called 1 shell), followed by L shell , M shell, and so on farther and farther from the nucleus. The chemical properties of the atom are determined by the electrons in the outermost occupied shell. The outermost shell is called the valence shell.
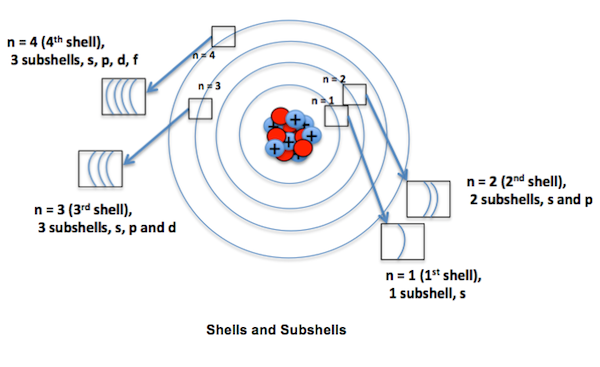
The principal quantum number (n) determines the maximum number of electrons that can be accommodated in a shell. With the increase in the value of ‘n’, the number of allowed orbital also increases and is given by ‘n2 ‘. All the orbitals having a given value of n constitute a single shell of atom. Size of an orbital will increase with increase of principal quantum number ‘n’. In other words the electron will be located away from the nucleus. Since energy is required in shifting away the negatively charged electron from the positively charged nucleus, the energy of the orbital will increase with increase of n. It is represented by the formula 2n2, where ‘n’ is the shell number. The shells, values of n, and the total number of electrons that can be accommodated are tabulated below.
| Shell and ‘n’ value | Max. Electrons in the Electronic Configuration |
| K shell, n=1 | 2*12 = 2 |
| L shell, n=2 | 2*22 = 8 |
| M shell, n=3 | 2*32 = 18 |
| N shell, n=4 | 2*42 = 32 |
Subshells
Each shell consists of one or more subshells or sub-levels.
- The subshells into which electrons are distributed are based on the azimuthal quantum number (denoted by ‘l’).
- This quantum number is dependent on the value of the principal quantum number, n. Therefore, when n has a value of 4, four different subshells are possible.
- For example in the first shell (n = 1), there is only one sub-shell which corresponds to l = 0. There are two sub-shells (l = 0, 1) in the second shell (n = 2), three (l = 0, 1, 2) in third shell (n = 3). Similarly when n=4. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells respectively.
- The maximum number of electrons that can be accommodated by a subshell is given by the formula 2*(2l + 1).
- Therefore, the s, p, d, and f subshells can accommodate a maximum of 2, 6, 10, and 14 electrons respectively.
All the subshells which are possible for values of n up to 4 are given below.
| Principle Quantum Number Value | Value of Azimuthal Quantum Number | Resulting Subshell in the Electronic Configuration |
| n=1 | l=0 | 1s |
| n=2 | l=0 | 2s |
| l=1 | 2p | |
| n=3 | l=0 | 3s |
| l=1 | 3p | |
| l=2 | 3d | |
| n=4 | l=0 | 4s |
| l=1 | 4p | |
| l=2 | 4d | |
| l=3 | 4f |
As the value of the azimuthal quantum number is always less than that of the principal quantum number, it can be understood that 1p, 2d, and 3f orbitals do not exist.