Phenols are organic hydrocarbon compounds with a hydroxyl group directly attached to an aromatic hydrocarbon compound like Benzene Ring. Phenols are also termed as Phenolic compounds. The molecular the formula of phenols is C6H5O6. The synthesis of Phenols is Natural as well as artificial.
Electrophilic substitution reaction is one of the most important chemical properties of phenols. The phenols are compounds in which the hydroxyl atom is attached to the aromatic Benzene atom. The OH atom in the phenol is attached to the sp2 carbon atom of the benzene ring. The sp2 hybridized is highly electro negative in nature. As a result of this the electron density of the Oxygen atom of the phenols. This makes the phenoxide ion more attackable by an electrophile.
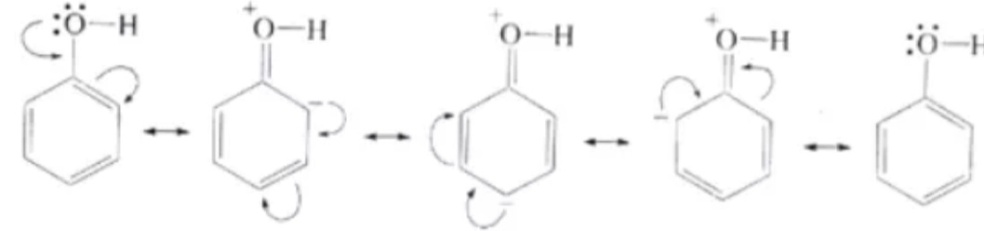
The rearrangement of electrons in the phenol compound is one of the reasons for the proper electrophilic substitution reactions. In the rearrangement of electrons in the phenols, the electron cloud gets rearranged and shifted to the sp2 hybridized carbon atom of the benzene ring. This is because of the higher electronegativity of the sp2 hybridization assays of the carbon atom. This result in a double bond formation between the oxygen and carbon atom. This is because of the electron cloud being transferred from the oxygen. This, there is a possibility of forming the double bond and getting stabilized subsequently.
There are a number of electrophilic substitution reactions carried out by the phenols. They are as follows:
Kolbe’s reaction.
The kolbe’s reaction involves the reaction of phenol with NaOH and formation of phenoxide ion. This phenoxide ion is further treated with acid. This leads to the electrophilic substitution process, and the end product is Salicylic acid.
Reimer Tiemann reaction.
This reaction involves the treating of phenol compound with chloroform(CHCl3) along with base (KOH). The end product formed by subsequent electrophilic substitution reaction is salicylaldehyde.
In the above reaction if we may use CHCl4 instead of CHCl3 then the final product obtained will be salicylic acid.
Nitration of phenols.
Nutrition of phenols involves the electrophilic substitution reaction. When the phenols are reacted with conc. HNO3 acid then the phenol compound gets substituted with NO2 at 2, 4, 6 carbon atom of the aromatic ring. Thus, the final product is 2,4,6 trinitrophenol.
In order to get mono substituted phenol, we must use dil. HNO3 acid.
Bromination of phenol.
The monobromination of phenol can be done by the reaction of Bromine along with non polar solvents like CS2 or CCl4. The final product is a monobromo phenol.