The Electromotive Force or the EMF, measured in coulumbs of charge. This pressure is developed or the electrical intensity or from electrical source or energy.
It’s a device that converts any energy form into electrical energy that is later measured with coulumbs of charge. EMF which is Electromotive Force is denoted by, ℰ.
So, emf = I (R + r)
Where I = current in A,
R = Resistance of load in a circuit (ohms);
r = Internal resistance (ohms)
emf = E/ Q
Where,
E = Energy (joules),
Q = Charge in coulombs.
Internal Resistance
When the current exists in a device or an electrical circuit, then there’s a drop in the voltage in the source voltage, or the source battery is the internal resistance. The reason of this occurrence is because of the electrolytic material present in batteries or any other voltage sources.
Internal Resistance (r) = (E – V)/I
Where,
E = emf of the device,
V = potential difference between the device,
I = current in the device,
Internal Resistance is the conclusion of resistance acting in the battery or the gathering in the battery.
The equation used for derivation is follows as:
V = (E – Ir)
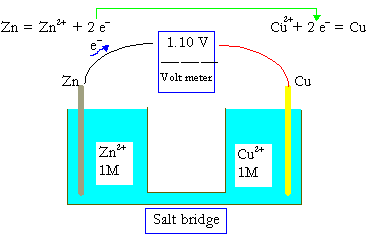
The EMF is defined as the maximum (V) potential difference in between 2 electrodes belonging to a voltaic or the galvanic cell.
This quantity has the relation with the element’s tendency, an ion or compound to release (lose) or gain (accept) electrons. Let’s say, the maximum potential difference between
Cu and Zn of a popular cell
Zn(s) |Zn2+ (1M) | |Cu2+(1M) | Cu(s)
Which is measured in the value of 1.100 V. The concentration of one M in the ideal solution is known as the standard condition, and thus, 1.100 V is called the standard cell potential , or the standard electromotive force, DEo for the Zn−Cu galvanic cell.
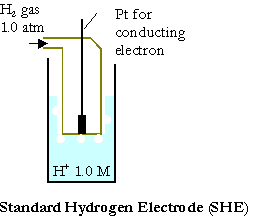
The standard cell potential or the standard electromotive force, DEo, of the given galvanic cell can be calculated from the standard reduction potentials of 2 half-cells “Eo”.
The measurements of reduction potentials are done on contrary to (SHE), standard hydrogen electrode:
Pt(s) | H2(g,1.0atm) | H+(1.0M)
Its oxidation potential or the reduction potential is determined to be zero exactly.
All other half-cells having the reduction potentials are measured in V (volts) opposite to SHE, which are the electric potential difference energy per coulomb of charge.
Remember, that energy unit is J = Coulomb volt,
Gibbs free energy = G and,
G = product of (E) potential difference and q charge,
G in Joules = q E in C V, for the calculations of electric energy