Alcohols are prepared by the various method including the reduction of aldehydes, acids, esters, and ketones or by the hydration of the alkenes, Grignard reaction with aldehydes, and ketones, reduction of esters and carboxylic acids, from the hydrolysis of alkyl halides and by the fermentation process.
Hydration of Alkenes
Elements of water are added to the double-bonded carbons of the alkenes and alcohol is obtained. Among the primary alcohols only ethyl alcohol can be obtained by this process. Primary alcohols are obtained by the hydroboration of terminal alkenes.
Reduction of Aldehydes and Ketones
The reduction of ketones and aldehydes yields the alcohols and the reduction conditions are the same for reducing the alkenes’ double bonds. Sodium borohydride is the best option for reduction if a molecule has both double bonds and a ketone or aldehyde. When cyclohexanone is reduced by the hydrogen gas along with the use of platinum catalyst it produces the good yield of the cyclohexanol.
Grignard Reaction with Aldehyde and Ketones
Grignard reaction is the simplest method for producing the primary, secondary, and tertiary alcohols. Grignard reaction is made to react with the formaldehyde for the production of primary alcohol. And the reaction of the Grignard reagent with another alcohol leads to the formation of a secondary alcohol.
Reduction of Esters
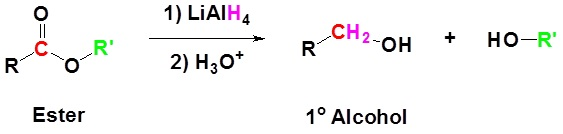
Esters such as the carboxylic acids are reduced with the lithium aluminum hydrides and two alcohols are formed in this reaction. The reduction of methyl benzoate to the methanol and benzyl alcohol is the example of the reduction of esters.
Reduction of Carboxylic Acids
By the reduction of carboxylic acids, primary alcohol is formed. For this reduction, a strong reducing agent is required and lithium aluminum hydride is the best choice for this reaction. Carboxylic acids are also reduced to the alcohols by the action of diborane. By the catalytic hydrogenation, poor yields are obtained and it is not used commonly, for such reactions.
From Haloalkane
The reaction of aqueous alkali with the haloalkanes yields the corresponding alcohols.
From Hydrolysis of Alkyl Halides
The reaction of hydrolysis of alkyl halides is the nucleophilic substitution reaction. However, this is not widely used due to the production of olefins as byproducts.
By the Fermentation
In the fermentation process, complex organic compounds are slowly decomposed to the smaller and simpler ones by the enzymatic activities. Generally, this process is exothermic in nature and is accompanied by the evolution of methane and carbon dioxide gasses. In the alcoholic fermentation, sugar is converted to ethyl alcohol by the action of yeast. The use of starting material depends on availability in different countries.