Chemical thermodynamics: Standard Enthalpy of Combustion
Combustion is a chemical reaction that occurs between any fuel and oxygen. Heat energy is produced from the combustion of a fuel with air. The internal energy of the fuel species is their chemical energy. This energy corresponds to the chemical bonds and intermolecular attractions. During a chemical reaction, some chemical bonds are broken, and some are formed with the transfer of heat energy between the system and its surroundings.
The heat of combustion is defined as the quantity of heat evolved in the combustion of a fuel with a full supply of oxygen. For quantitative analysis of combustion, some important properties such as, pressure, volume, temperature, internal energy, enthalpy, entropy and free energy are required to be analyzed. The properties depend on the final and initial conditions of the system, are called state functions and they are independent of the path taken between the initial and the final state. The state function related to a chemical reaction system such as combustion is its enthalpy. Enthalpy is evaluated at standard conditions, 1 atm pressure (101 kPa) and a temperature of 298 K. The enthalpy of an element at standard conditions is designated to be zero.
Likewise, Standard Enthalpy of Combustion refers to the complete combustion of one mole of the substance in oxygen under standard conditions (298K and 1 bar pressure). For example:
H2(g)+12O2(g)→H2O(l);ΔHc° = −286kJmol−1
C4H10(g)+132O2(g)→4CO2(g)+5H2O(l); ΔHc° = −2658kJmol−1
Enthalpy of the fuel and oxidant are relative to that of the enthalpy of the combustion products. Upon combustion in oxygen, hydrocarbon fuels produce carbon dioxide and water with the release of heat energy.
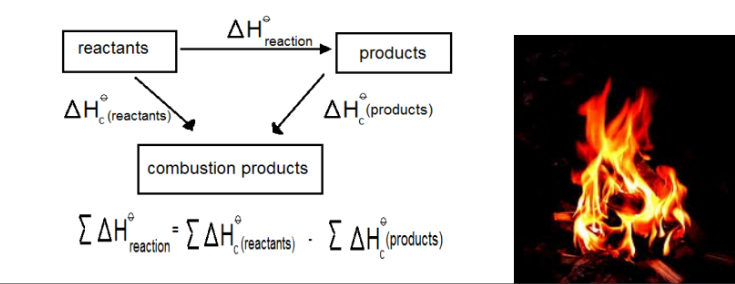
The standard enthalpy of change of a reaction can be calculated using Hess law and the standard enthalpy changes of combustion in cases where both sides of the equation can be burned in oxygen. Hess’s law states that the total amount of heat released or absorbed in a chemical reaction remains the same regardless of whether the process takes place in one or more steps.
The following example illustrates the Hess’s law in the combustion of a fuel:
The reaction can follow two routes:
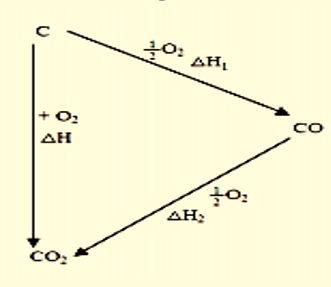
Figure 1: Routes for the burning of pure carbon
- Direct burning of carbon to carbon dioxide.
$C(s)+O_{2}(g)\to CO_{2}(g); \Delta H=-393.5 kJ/mol$ - Carbon burning in insufficient oxygen followed by further oxidation of carbon monoxide.
$C(s)+\frac{1}{2}O_{2}(g)\to CO(g); \Delta H_{1}=-110.4\frac{kJ}{mol}$$CO(g)+\frac{1}{2}O_{2}(g)\to CO_{2}(g); \Delta H_{2}=-283.2 kJ/mol$
Overall enthalpy change =
$\Delta H_{1}+ \Delta H_{2}=-110.4-283.2=-393.6 kJ/mol$
An observation can be made that the standard enthalpy of combustion of carbon (C(s) + O2(g) → CO2(g)) is the same as the standard enthalpy of formation of carbon dioxide. Since it is the same reaction and all the reactants and products are in standard states in both equations. Note that the combustion must be per mole of substance burned the same as with all standard combustion reactions and enthalpy values. Hence, if 2 moles of a substance is combusted then the standard enthalpy of reaction is the standard enthalpy of combustion multiplied by 2.
Most elements and compounds combine with oxygen. These oxidation reactions are highly exothermic which makes the measurement of their heats relatively easy. At a laboratory scale and for other experimental purposes, a bomb calorimeter is used to determine the heat of combustion.