These aromatic hydrocarbons are well known due to their exceptional chemical properties and their chemical properties are listed below.
- The aromatic hydrocarbons exhibit the aromaticity that is additional stability which is granted by the resonance. In the aromatic hydrocarbons, the ratio of carbon atoms to hydrogen atoms is high. When they are burnt, they display a sooty and strong flame of yellow color. Generally, these compounds undergo nucleophilic aromatic substitution reactions and electrophilic substitutions. These compounds can be either polycyclic or monocyclic.
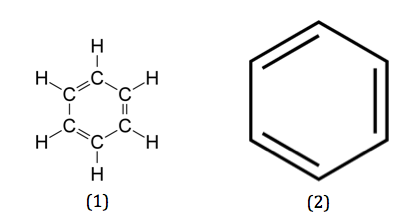
- Aromatic hydrocarbons have the closed rings of the alternative single and double bonds with the delocalized electrons.
- The aromatic compounds having at least one conjugated ring of single and double bonds exhibit the extreme stability.
- These compounds are cyclic where ring atoms are participating in the network of the pi bonds and resultantly, they show unusual stability.
- Aromatic hydrocarbons undergo various reactions including, aromatic substitution reactions, coupling reactions, and hydrogenation reactions. Aromatic compounds have double bonds but it is less likely to undergo the addition reactions than the double bonds of the typical alkenes. On the aromatic compounds, the order of the substitution is governed by the nature of the substituents which are present in the aromatic rings.
- Due to their chemical properties, their origin dates back to coal and petroleum. They can also exist in the form of the polyaromatic hydrocarbons which contains more than one benzene. Their escape to the atmosphere creates pollution and they have potentially negative effects on all forms of life as they are carcinogenic.
- Precursors to the nucleotides and amino acids all are the aromatic compounds. The aromatic hydrocarbons do not make the hydrogen bonds or ions with the water molecules. Due to extra stability, they are unreactive for many inorganic and organic reactions so they are widely used as inert solvents. As compare to the alkenes, their reactivity is much less so they are useful industrial solvents for the non-polar compounds.
- The ratio for carbon to hydrogen in the aromatic compounds is high. Due to the presence of the high carbon content, they burn with the sooty flame of yellow color.
- The aromatic hydrocarbons having the multiple bonds are unsaturated in nature like alkynes and the alkenes. Due to unsaturation, they tend to give addition reactions.
- Due to electrophilic substitution reactions and resonance, the aromatic hydrocarbons are stable. In these reactions, carbon ring acts as a nucleophile and make a substituted product due to the electrophilic attack on the benzene.
- One of the hydrogen atoms of the ring is substituted with the coming electrophile and the product also holds the aromatic properties and stability in nature.
- During the addition reactions, the aromatic hydrocarbons may lose their aromaticity so, such types of reactions are not preferred by these compounds.
- The network of pi bonds is connected so the rings are planar in the aromatic hydrocarbons.
- Usually, the aromatic compounds are best represented by the continuous electron density that is evenly distributed on the aromatic core. They have a higher activation barrier due to the force of aromaticity that causes stabilization.