The conductor is usually a substance in which there is a property of passing various types of energy. Following is the explanation of band theory and electrical conductivity in conductors.
Metallic bonding: (Fermi gas) or Free valence electrons and fixed ions
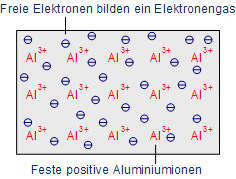
Conductivity is dependent additionally on temperature, the difference. If the temperature increases, the movements of atoms in the metal is even stronger so that electrons are forced or strained in their motions or movements. As a result, the resistance rises up. (Au) Gold and (Ag) Silver are the best conductors of electricity and they are used comparatively rare due to their expensiveness (like gold). So, in semiconductors, the alternative components used in microchips are – (Al) Aluminium and (Cu) Copper.
Metals such as copper (Cu), aluminum (Al) are having no forbidden gap resent in between conduction band and valence band.
Here, the valence band and conduction band overlap. Therefore, even at the room temperature, a huge electron number is available for the conduction.
Thus, with the use of any extra or additional energy, these metal types are having a great number of free electrons and accordingly known as the good conductors.
| ELEMENT | ∆E(kJ/mol) of energy gap | # of electrons/cm^3 in conduction band | insulator, or conductor? |
| C (diamond) | 524 (big band gap) | 10-27 | insulator |
| Si | 117 (smaller band gap, but not a full conductor) | 109 | semiconductor |
| Ge | 66 (smaller band gap, but still not a full conductor) | 1013 | semiconductor |
The below equation is probablity to find out the electron residing in conduction band, which is given by:
P=1eΔE/RT+1……………….Eq 1
Where,
∆E = Equation for change in energy gap or energy,
t = Stands for temperature,
and R = Bonding constant.
This equation and the above table below show that how the big the difference is in the energy gap or energy or gap, between conduction band and valence band. The probability of less electrons are seen in conduction band. This is the reason why they can’t be excited or incited that much to go for a jump to conduction band.
Atoms’ energy bands are in interdependent with different atoms
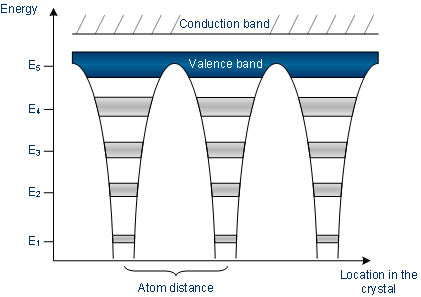
The band model of conductors
In a conductor, either the valence band is not fully filled with electrons, or the “filled valence band” along with vacant conduction band overlaps.
Generally, both states takes place simultaneously, therefore, the electrons can move within partially filled (V.B) valence band or within the bands which overlap.
No band gap is there in between conduction band and the valence band in a conductor.
Or,
You can understand in this manner that,
In a conductor case, the final energy levels’ band occupied is just partially filled. The lowest levels are occupied by the possible electrons one at a time as per “Pauli’s exclusion principle.”
This leave behind a portion of this band, known as the conduction band which is unoccupied.
In valence band, electrons freely move in conduction band which is fileld partially.
In the conduction band which is partially filled, the topmost energy level is occupied at absolute zero temperature by electrons, which is referred to as the “Fermi level” and the equivalent energy is called the “Fermi energy.”

Energy bands in conductors Energy bands in insulators