The alkanes are the hydrocarbons that are completely saturated and exist in the acyclic or cyclic type of structures. Generally, all the compounds ending with ane are known as alkanes. The physical properties of alkanes very for a different number of carbon atoms present in the parent chain or the cyclic compound.
Alkanes are hydrocarbon compounds that have mainly C-C and C-H type of bondings. Due to such bonds, the compounds are almost none polar in nature. This is due to the existence of very weak difference of electronegativity between the C and H atoms. Hence the alkane has weak Vanderwaal forces as interpersonal forces between them. As a result of such weak interparticle forces the different number if carbon chained alkanes exhibit a different state of nature. The C1 to C4 carbon atom alkanes are gaseous in nature. That means the alkanes methane, ethane, propane, and butane exist in the gaseous state. Whereas from the C5 to C17 carbon chained alkanes exist in the state of liquid. This could be understood as the butane compound is gaseous in nature but the pentane hydrocarbon compound is liquid in nature. The rest of the compounds that have more than 17 carbon atoms in their chain are waxy solid in nature. Due to such properties of alkanes of the hydrocarbon compounds of methane, ethane, propane and butane are used as gas petroleum in CNG and LPG. Whereas the compounds above butane are used as lubricant fluids and greasing agents at several sites.
Boiling Points
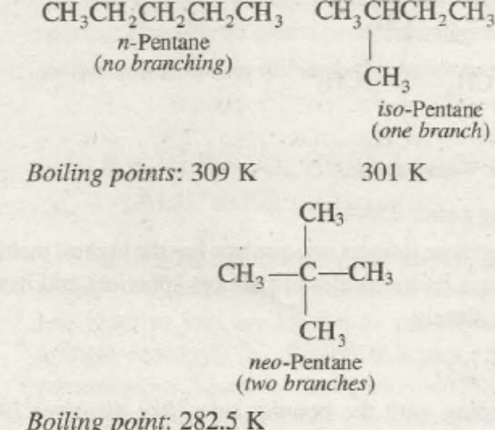
The boiling point of alkane hydrocarbons is also one of the varying physical property of alkanes. The alkanes, in general, have low boiling points. But as the number of carbons in the n-alkanes increases the boiling point of the alkanes also rises. This is due to an increase in the molecular weight of the compound. We know that as and when the molecular weight of the n-type alkanes increases the Vanderwaal interparticle forces between the particles also increases. As a result of the increase in the interparticle forces, it becomes difficult to separate the particles and thus increasing the boiling point of the compound subsequently. For the branching compounds as and when the branching increases the boiling point of the compound also decreases. It is as a result of decreases in the surface area of the compound and decreasing the London dispersion forces among the particles of the compounds.
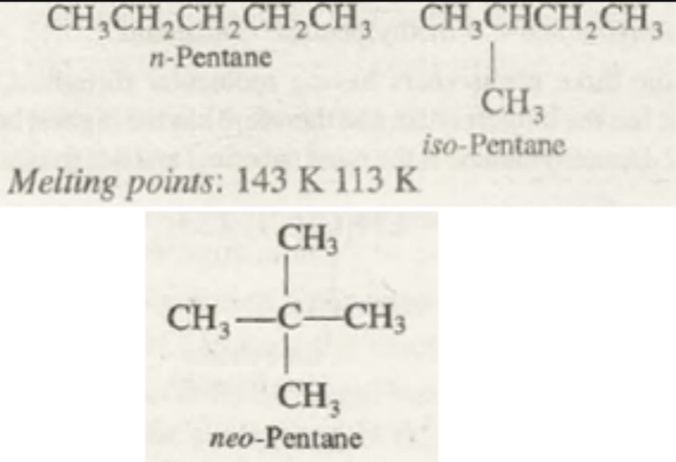
Melting point
The melting point of the alkanes is one of the dynamic physical property of alkanes. From different experiments and observations, the following is concluded. There is a relatively higher increase in the melting point of the alkane while moving from an odd-numbered carbon compound to higher carbon compound. Whereas the increase in the melting point while moving from an even numbered carbon compound is lesser while moving to a higher compounds of alkanes.
Volatility
The volatility of a compound is the ease with which it gets converted to vapours. For lower alkane compounds the volatility of the hydrocarbons is quite high. Whereas with an increase in the chain length of the compound the volatility decreases.
Solubility
In this physical property of alkanes, it is dependent on the fact that whether the solvent used for dissolving the alkanes are polar or nonpolar in nature. For the alkanes the nonpolar solvents like ether are perfect for dissolving them.