Dehydration of alcohol causes the production of alkenes. It happens by the process in which alcohol undergoes the E1 and E2 mechanisms and to make double bond and to lose the water. Mainly this reaction produces the alkenes and this process is proceeded in the presence of strong acid, by heating the alcohols. For this purpose, sulphuric acid and phosphoric acid may be used at higher temperatures. The range of the reaction temperature that is required is generally decreased with an increase in the substitution of the hydroxy-containing carbon. If heat is not sufficient during the reaction, then alcohols are not dehydrated and no alkenes are produced but instead, the alcohols react with each other and form the ethers.
Alcohols are amphoteric in nature and they can act as both base and acid. The oxygen atom has the lone pair of electrons and makes the hydroxyl group that is weakly basic. This oxygen has the capability to donate these two electrons to an electron-deficient proton. There are slightly different pathways and mechanisms for the dehydration of different alcohols. However, the main concept for the dehydration of the alcohols is the same that is the hydroxyl group in the alcohol donates its two electrons to the hydrogen from the acidic reagent and form an alkyloxonium ion. This ion is a very good leaving group that leaves to make a carbocation. Then the base which is deprotonated acid reacts with the hydrogen that is adjacent to the carbocation and makes a double bond.
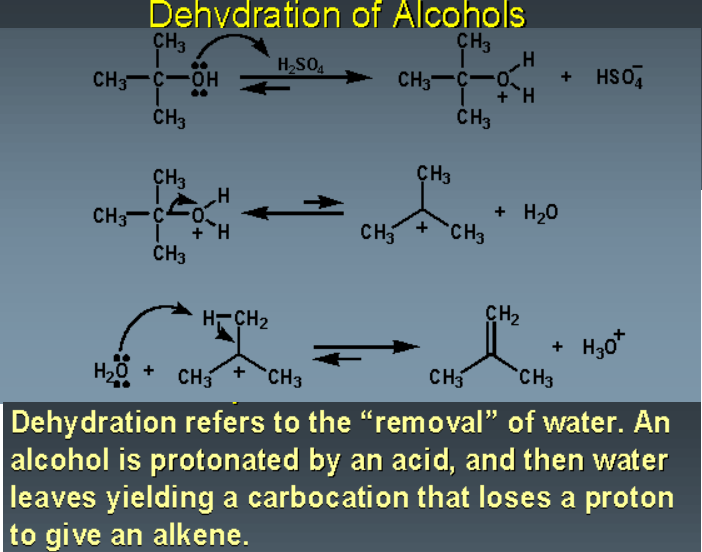
Primary alcohols undergo the E2 mechanism of dehydration. Here the oxygen of the hydroxyl group donates its electron pair to the proton from the sulphuric acid and make an alkyloxonium ion. In the next step conjugate base of the sulphuric acid reacts with the beta hydrogen atom that is adjacent to it and in the concerted process alkyloxonium, ion leaves and a double bond is formed. Secondary and tertiary alcohols follow the E1 mechanism of dehydration and similar to the reaction for primary alcohols their hydroxyl group protonates and make the alkyloxonium ion. However, for secondary and tertiary alcohols the ion is leaving first and makes carbocation as a reaction intermediate. In the next step, the water molecule abstracts the proton from the adjacent carbon and makes the double bond. For tertiary alcohol, the dehydration mechanism is analogous to the dehydration mechanism of the secondary alcohol.