As the covalent bond progresses ionic nature because of the electronegativity differences of atoms which are bonded (atoms having chemical bonds), even the ionic bond progresses covalent nature as explained below:
Distortion of the electron cloud of anion

When the charged ions of opposite nature come close to each other, the positive ion likely to draw the attention of ‘electron cloud’ or ‘atomic orbital’ of a negative ion approaching towards itself as the atomic orbital present in the anion (negatively charges ions) is held loosely by the means of its nucleus. Not for a long time, the anion remains spherical but certainly goes through some distortion. Anion’s electron cloud obtains the state of polarization and the electron density is drawn between two ions’ nuclei.
The arrangement of the electron density (e− density) present between two nuclei is shown in the above figure. In this, the ionic bond is not able to show 100
- Cation power causes distortion in the atomic orbital or electron cloud in an informal way, of the anion, is known as its “Polarising Power.”
- Anion’s ability to develop into a polarized state with the help of cation is known as its “Polarizability”.
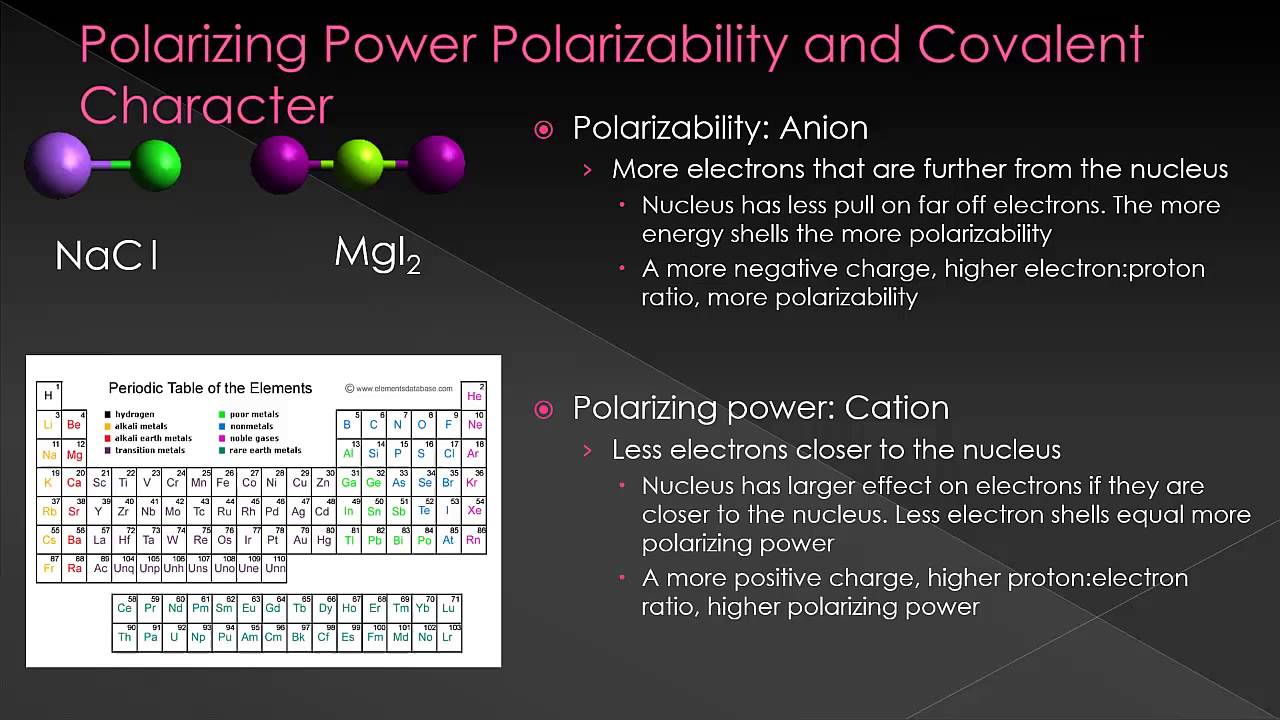
- The magnitude of the covalent character of ionic bond totally depends on cations’ polarising power and anion’s polarizability which are determined on the basis of “Fajan Rules”. These rules are explained below:
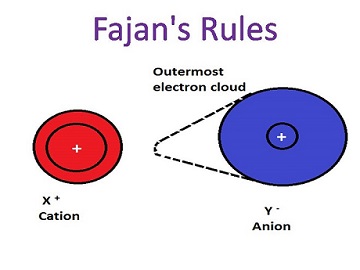
1. Cation and polarising power are indirectly proportional to each other, as larger the polarising power, small is the cation size. Also, cation’s polarising power is dependent on the electric field which is used to pull or attract electrons away from negatively charged ions (anions). The strength of the electric field is dependent on the charge density, represented by, q/r which means q is the charge and r is the size. So, cations’ polarising power and charge density go hand-in-hand as higher the polarising power, larger is cations’ charge density. Let’s say, compare Na+, Ma2:+” and A13+ ions’ polarising power
Na+ Mg2+ AI3+
Charge (q): + 1 + 2 + 3
Size (r) (nanometre, nm): 0.102 0.072 0.053
q/r ratio: 9.8 27.8 -56.6
Hence, you can easily see that polarising power maximize from Na+ à Mg2+ à AI3+.
Therefore, covalent character accelerates amongst their specific chlorides as NaCl < MgCl2 < AICl3.
2. Considering two cations of the identical size, one cation with “pseudo noble gas configuration” having the configuration, ns2np6nd10 consist of greater polarization power compared with those with “noble gas configuration” having configuration, n2p6. Let’s say, CuCI is having higher covalency than NaCI, the reason is – the polarization power of the Cu + ion, which features pseudo noble gas configuration is much more than the Na + ion.
3. Greater anion size, higher is its polarizability
In fact, anion polarizability is dependent on “qr” product. Greater the value of product qr of anion, more advanced will be its “polarizability”.
Let’s say, the contrasting difference of polarization of F-, Cl- and I- ions are mentioned below:
-F Cl- I-
Charge (q): -1 -1 -1
Size (r) (nm): 0.136 0.181 0.216
(qr) product: 0.136 0.181 0.216
As clear from above, the product qr grows from p- to I- ion (in this place, in magnitude, only 15 is taken). Hence, polarizability becomes higher as in – m p=- tO Cl- to I-.
Therefore, covalent character expands from MgF2 to MgC12 –MI2