General Introduction
Organic Chemistry is a branch of chemistry that deals with the structure, properties and reactions of organic compounds. Earlier it was thought that compounds which are obtained from plants and animals are organic compounds and compounds. And the compounds which are obtained from minerals; non-living sources are termed as inorganic compounds. The modern definition of organic compound is different than this.
Organic compound is any compound containing carbon and hydrogen or is a derivative of it. It consists of carbon in covalent bonding. The element carbon has the unique property of catenation. Due to this it forms covalent bonds with other carbon atoms. Along with this it also forms covalent bonds with atoms of other elements like hydrogen, oxygen, nitrogen, sulphur, phosphorus and halogens.
Organic compounds are essential for sustaining life on earth. It includes complex molecules like genetic information bearing DNA, proteins constituting essential part of blood, muscles and skin. Organic chemicals are used making of materials like clothing, fuels, polymers, dyes and medicines. These are few of the important areas of application and uses of these compounds.
Organic chemistry as a branch of science is about two hundred years old. In around late 18th century, scientists started to distinguish between organic compounds obtained from plants and animals and inorganic compounds prepared from mineral sources. A Swedish chemist,Berzelius came up with a theory that a ‘vital force’ was responsible for the formation of organic compounds. This notion was however rejected in 1828 when F. Wohler synthesised an organic compound by using an inorganic compound, ammonium cyanate. The development of the theory of covalent bonding,brought a new era for organic chemistry.
Common or Trivial Names of Some Organic Compounds
The general characteristics of Organic Compounds include:
- Organic compounds can be isolated as well as prepared in laboratory
- They comprise almost 90
- They have complex structures and high molecular weights
- Properties of organic compounds are decided by certain active atom or group of atoms known as the functional group.
- They are mostly insoluble in water but soluble in organic solvents.
- They are combustible in nature.
- Chemical reactions which involve organic compounds are slower in rate.
Characteristics due to Presence of Covalent Bonds
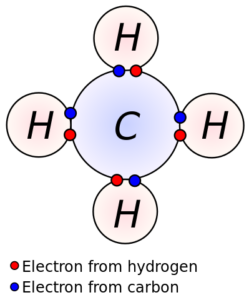
A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms that result in a stable balance of attractive and repulsive forces between the atoms. The presence of a covalent bond gives certain characteristics to the organic compounds. These include:
- Low boiling points and melting points in comparison to the inorganic compounds.
- Organic acids and bases are less strong and thus they have a limited dissociation in an aqueous medium.
- Phenomenon of isomerism is exhibited by them. In this a single molecular formula represents several organic compounds differing in physical and chemical properties.
- They are volatile in nature.