Chemical Kinetics: Factors affecting the rate of reaction
Concentration
Concentration is the amount of a substance (solute) present in a given volume of solution. Therefore, a more concentrated solution results in an increased number of molecules that collide more, thereby increasing the rate of reaction. Most chemical reactions rates increase when the concentration of one or more of the reactants is increased. Likewise, cramming more particles into a fixed volume increases the concentration of reactants, and, thus, the frequency of collision.
In some reactions, however, the rate is unaffected by the concentration of a reactant, as long as it is present at some concentration. In such cases, the nature of reactant is the limiting factor that decides the direction and rate at which the reaction proceeds.
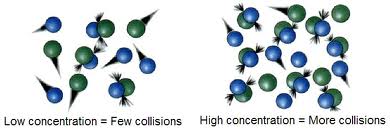
Fig 1: Effect of concentration on the reaction rate
For example, a lighted splint glows in air and soon dies out because air is only 20
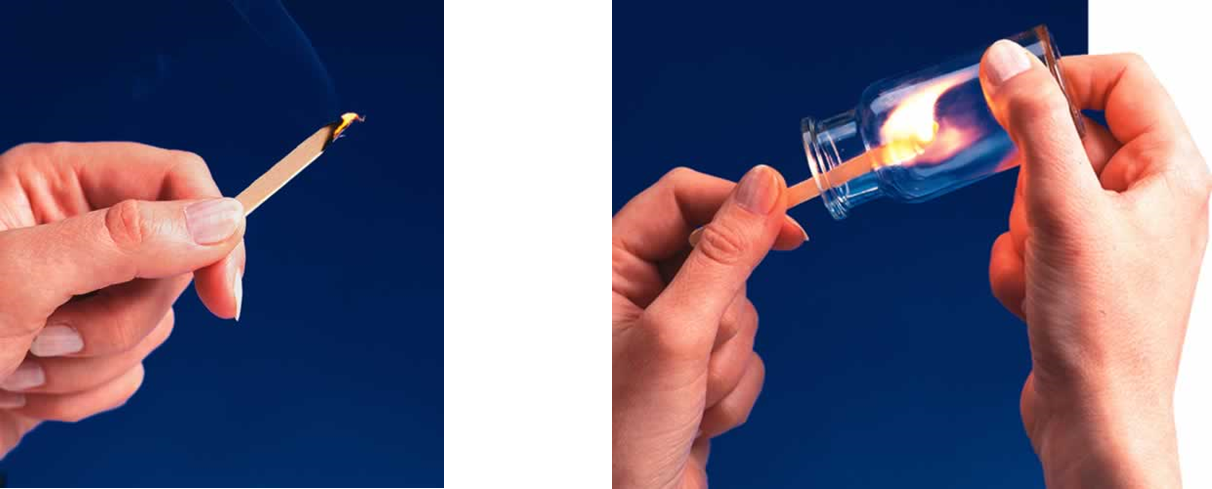
Fig 2: Lighting a splint in air and pure oxygen respectively.
Consider the reaction between zinc and hydrochloric acid:
Zn + 2HCl H2 + ZnCl2
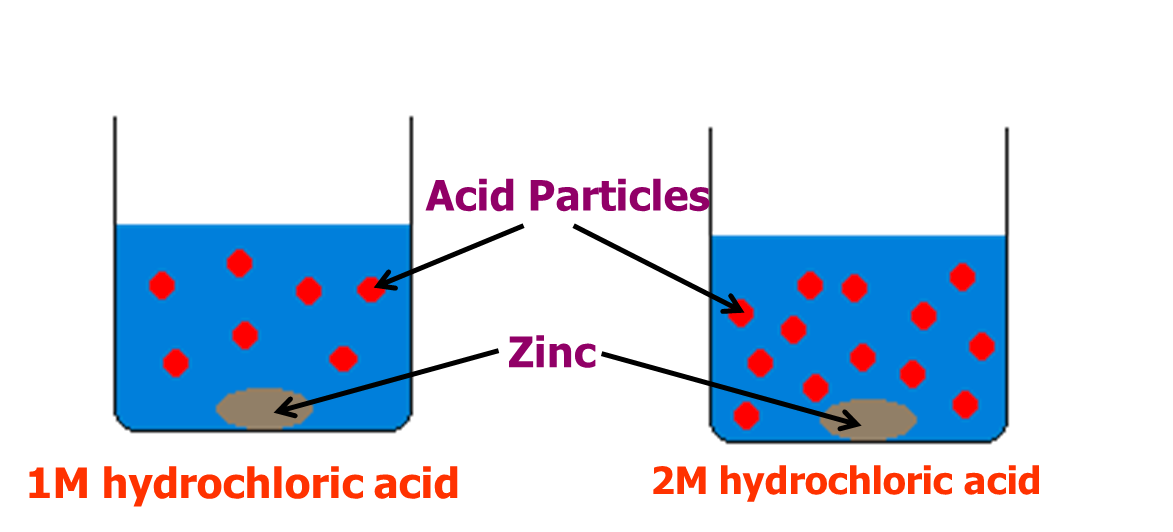
Fig 3: Reaction between Zinc and hydrochloric acid.
In this example, more particles of acid per unit volume in the 2M acid are observed than the particles in the 1M acid. Likewise, the collisions between the acid and zinc particles in the stronger acid will more than the weaker one giving a faster reaction rate.
Similarly, the rate of reaction between gases is increased by increased pressure.As per the universal gas law, the pressure is the gas equivalent of concentration.
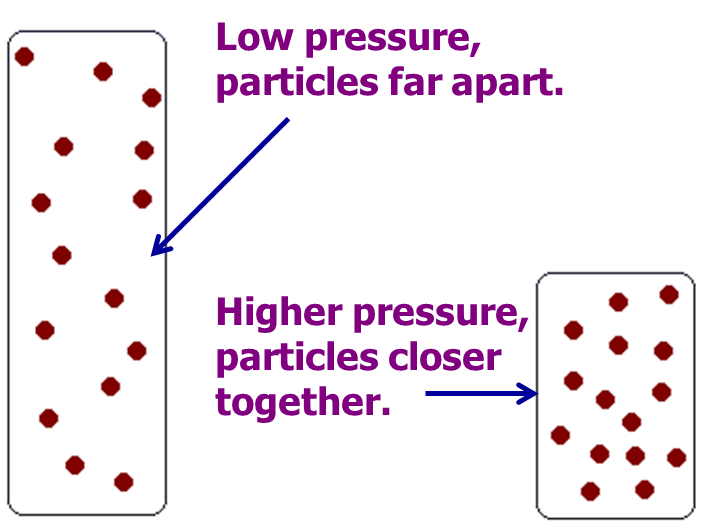
Fig 4: Dependence of reaction rate on concentration in gases.
In the figure above, the two gas jars contain the same number of gas particles. The jar with higher pressure has more particles per unit volume confirming a higher concentration, hence faster reaction.
Consider the following example
2N2O5(g) 4NO2(g)+ O2(g)
Notice that for every 1 mole of O2 that appears, 4 x as many moles of NO2 will also appear. In the meantime, twice as many moles of N2O5 will be disappearing as moles of O2 forming.Changes in concentrations of the reactants and/or products are inversely proportional to their stoichiometric proportions. This means that the rate of the reaction could be written as:
Rate = −½ ∆ [N2O5]/∆t = ¼ ∆[NO2]/∆t = ∆[O2]/∆t
Notice the negative sign on the rate of [N2O5] reminds us that it is disappearing.
The rate law can be used to show the relationship between the reaction rate and the concentrations of reactants. It is an equation that relates the rate of a reaction to the concentration of reactants (and catalyst) raised to various powers.
Consider the reaction of reactants A and B to give products D and E.
![]()
The rate law is written as:
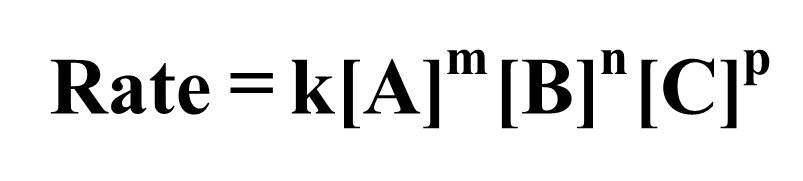
The exponents m, n, and p may or may not be integers. They must be determined experimentally and cannot be obtained by simply looking at the balanced equation.The rate constant, k, is a proportionality constant that represents the relationship between reaction rate and concentrations.