In the primary alcohol, the carbon atom on which the hydroxyl group is attached should be directly attached to the one carbon atom. Primary alcohols contain a group of CH2OH. Usually, this group is located at the end of the carbon chain. For the identification of primary alcohol, a look is enough on the branching of the carbon on which the hydroxyl group is attached. The primary alcohol is having the hydroxyl group attached to the 1 carbon that is also known as the primary carbon. There are certain tests for the identification of primary alcohols. Some of the tests will be discussed in this article.
Lucas Test
This test is based on the difference with the reactivity of the primary alcohols with the hydrogen chloride. In this test, a primary alcohol is treated with the Lucas reagent. As a result, turbidity is produced and the reason is that in the Lucas reagent the halides of the substituted alcohols are immiscible. The time in which turbidity is achieved is noted and few observations are made. At room temperature turbidity is not produced for the primary alcohols but an oily layer is formed on heating. Upon reaction of an alcohol with the Lucas reagent rate of formation of turbidity helps in the identification of the alcohols. This test is only feasible for the alcohols, which are soluble in the Lucas reagent. In some aspects, it is good for the primary alcohols, as the alcohols having up to 6 carbons can be easily identified by using this test.
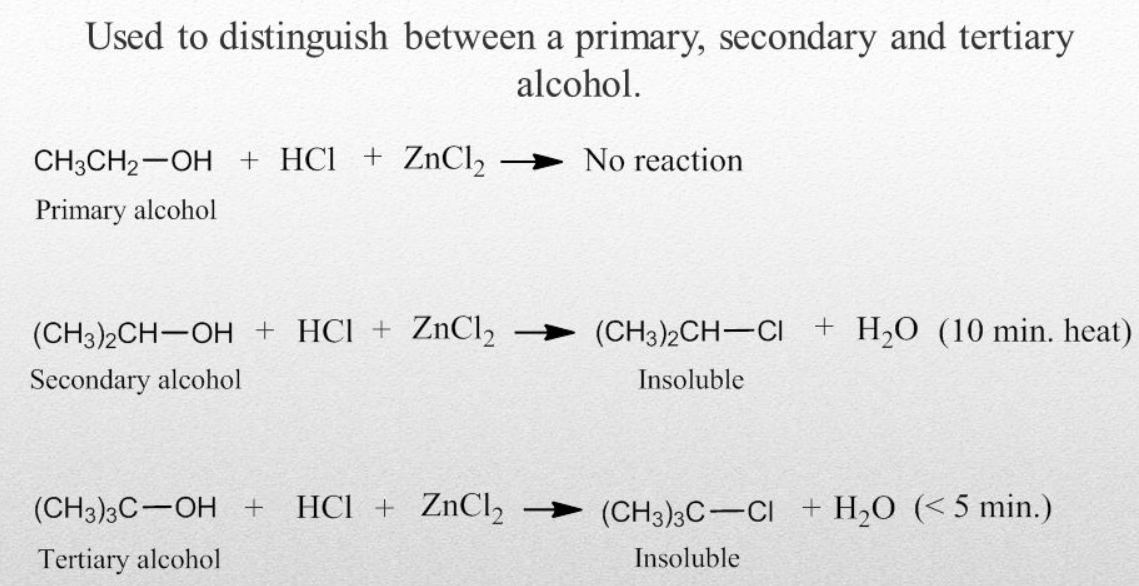
Oxidation Test
The oxidation of alcohols is done with sodium dichromate. The rate of oxidation is variable for different alcohols and based on the rate of oxidation alcohols can be distinguished and identified. Usually, depending on the conditions, primary alcohols are easily oxidized and gives aldehyde and the further oxidation of aldehyde gives the carboxylic acid. If an excess amount of alcohol is used then aldehyde is obtained and as soon as it is made it is distilled off. By oxidation, alcohols are converted to the ketones and aldehydes. Oxidation of ethanol causes the production of acetaldehyde and it can be identified by using the Schiff’s test which forms the magenta color immediately. Oxidation of methanol causes the production of formic acid and for its identification, solid sodium carbonate is added to the test tube having the green-colored solution after the pot.dichromate test and it will cause the effervescence.