The well-known relationships like Boyle’s law, Avogadro’s law, Charles’s law, they all can be mixed into an extremely useful formula called ideal gas equation, which is given by PV = nRT.
Or,
PV = nRT, known as an ideal gas equation, is used for the calculation of temperature, pressure, or volume, or no. of moles of a gas.
Where,
R = gas constant; “8.31 J K-1 mol-1”
P = pressure in pascals (Pa)
T = Temperature in Kelvin (K), the value of T in gas equations is always calculated in Kelvins
n = no. of moles
And the value of R is given by,
![]()
This formula is valid strictly for ideal gases only – in which the molecules are quite far so that the intermolecular forces can be ignored.
Under high-pressure conditions, these types of forces cause an important deviation from the ideal gas equation, and more complex equations are formulated to deal with such cases.
However, the ideal gas equation gives beneficial results for most of the gases at pressures not more than 100 atmospheres.
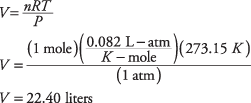
This is specific value is stated in the CO2 reaction; remember, 1 mole of any gas involves 22.4 liters of volume at STP.
An example on the above formula-
Question: Determine the value of n i.e the number of moles of gas contained in a bumpy house, having 20.63 cubic meters volume, 300 Kelvin temperature, and 101 KPa pressure.
Answer: Given:
P = 101 KPa
T = 300 K
V =20.63 cubic m
n = 8.31 J K-1 mol-1
And the formula says, PV = nRT
So, 101 × 20.63 = n × 8.314 × 300
2,083.63 = 2,493 × n
n = 0.83539 moles
0r 835.39 moles
What is STP and what do you mean by STP condition?
STP is abbreviated as ‘Standard Temperature and Pressure’. STP condition is described (according to International Standard Metric Conditions) as, where the neighboring absolute temperature is 288.15 Kelvin (15° Celcius) and the pressure is 1 atmosphere, that is 101.325 Kpa. or 1 bar, it’s is known as the STP condition.
So, from the Ideal Gas Equation, you should be able to determine one of out any four quantities – volume, pressure, temperature, or mole – if the values of other three are given to you.
Ideal Gases And Kinetic Theory
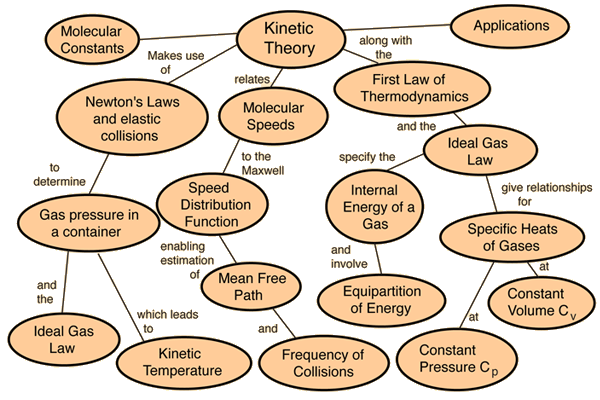
All the gases are based on the assumptions made by “Kinetic Theory of Matter”, which presumes that all matter is composed of particles (i.e. molecules or atoms); in between the particles, space is present, and the attractive forces evolve stronger as the particles get accumulated.
The particles follow random, constant motion, and the particles collide with each other and with the container’s wall in they are placed. Each particle has basic kinetic energy which depends only on temperature.
The above diagram is related to the kinetic theory of gases
It’s considered that there is an ideal gas and if its particles are known to be so far that they can’t apply any attractive force on each other. In actual life, an ideal gas is just nothing, but at low pressures and high temperatures, it behaves quite close to it. This is the reason why ideal gas law is a helpful approximation.