Percentage Composition
The percentage composition of any given compound is ratio of amount of every element to total amount of all elements present in compound. Then this value is multiplied by 100. This is mainly used to measure quantity in the terms of grams of elements which are present in solution.
In general percentage composition expresses the composition of an element in the terms of all elements present. It has significant importance in chemistry and is used for the chemical analysis as the chemical composition of various substances can be studied.
Calculation of Percentage Composition
The percentage composition of any given element can be expressed by using this formula.
![]()
This formula is based on the weight of each constituting element. Here gE stands for grams of elements, gT stands for total grams of a compound or molar mass of the compound.
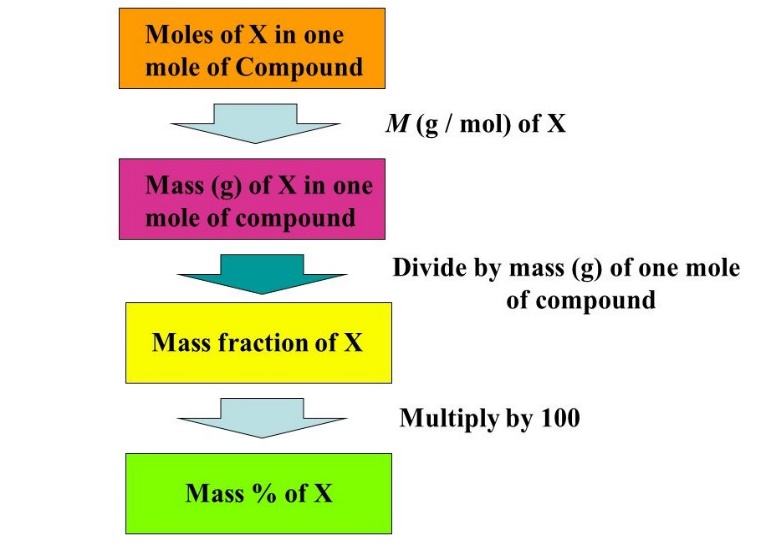
Following steps are involved to calculate the percentage composition.
- First of all, find molar mass of all elements in terms of gram per mole in a compound.
- In second step find molecular mass of entire compound.
- Then divide the molar mass of component by molecular mass of entire compound.
- A number having a value between 0 and 1 will be obtained then multiply it by 100 and percentage composition will be obtained.
Weight of each atom present in the compound and then percentage of total weight of all the molecules present in the mixture should be calculated. The percentage composition is percentage by the mass of each element in any given compound. For example, water has percent composition of 80
Importance of Using Percentage Composition
It is important to find out the percentage composition as it reveals the amount of certain substance in a compound. According to the law of definite proportions elements are always present in compounds in same proportions by mass and percentage composition is relative mass of each element in any compound. Percentage composition is a convenient way to describe the composition of atoms.
Empirical formula can also be driven by using the percentage composition which in turn is useful for finding the actual weight of molecule and molecular formulas for compounds which have covalent bonding. One can also calculate the mole and molar ratio of the elements present in a compound. After finding the molar ratio, the ratio of ions in compounds can also be find out. In this way it acts as starting point to find out the empirical formulas.
By using percentage composition one can easily determine that how much of a specific substance can be produced. However, read good lab practices thoroughly before working in lab to find the percentage composition and follow them for accurate results.