Conformations are basically the type of stereoisomers that shows the isomerism on the basis of rotations around the sigma bonds. This type of isomerism is termed as Conformational isomerism. The only barrier acting in the inter transitions of the isomers is the rotational energy. In order to change the conformations, the isomers have to overcome the rotational energy. The difference in rotational energy must be less enough so that the inter transition could be possible between the isomers. There are several conformations exhibited by the butane and ethane compounds.
Ethane is an organic hydrocarbon compound with two carbon atoms. The physical state of the ethane compound is a gaseous state. It is chemically active gas at a standard temperature. The gas is colourless. Also, there is no odour to the gas. The ethane molecule as such consists of seven sigma bond in all. The shape of the molecule changes. But due to the rotations there occur many possibilities.
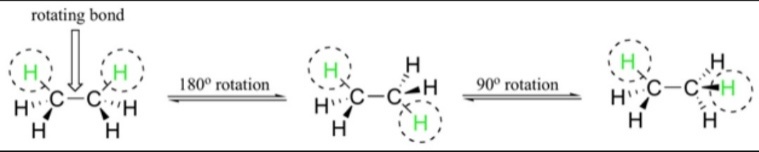
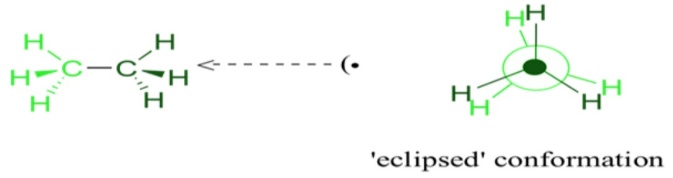
Now, let us rotate the CH3 group by 60°, there may be a possibility that the hydrogen present at the front carbon may get shifted to the back carbon position. This type of conformation is known as eclipse conformation. The eclipsed conformation is the highest conformation in the ethane compound. Once again when we further rotate the CH3 by 60° then we get the second eclipsed conformation. This is the second highest type of conformation in the ethane structure.
Several methods are used by researchers and chemists to determine the type of conformation of ethane. As it is difficult to evaluate the type of conformations new methods and processes are developed and researchers are still studying the improved methods to do so. This is because ethane is a gaseous state molecule. In the gaseous state, all the conformations of ethane coexist. As a result of this, it becomes difficult to segregate the conformations.
In order to properly understand the type of conformation present there is a method known as Wedges. In this method the dotted line is used to show the atoms out of the plane of the paper. Whereas the dark streak lines show that the atoms are going inside the plane of paper. The normal lines connecting the atom of ethane depicts the plane in which the paper lies.
The other method of drawing the conformations is the Newman projection method. The central carbon atom is represented with a dot on the paper. Then around the central carbon atom dot, a circle is drawn that depicts about the carbon atom at the back of the front carbon. The hydrogen and methyl atoms are connected to the carbon atoms as per their orientations in the planes of the atoms. The atoms are drawn at sixty degrees to each other. With different degrees of rotations infinite conformations are possible but in order to avoid such problems proper 60° rotations are considered.
Uses of ethane
The conformations of ethane are used in the manufacturing of ethane. It is mainly done by steam cracking method. It is majorly used in the ripening process of food. Also the main ingredient used is the mustard gas.