Surface Chemistry: Adsorption
Chemisorption
Chemical adsorption or chemisorption results from a chemical interaction between the adsorbate and adsorbent. Therefore, formed a bond (covalent, ionic, metallic) with bond energy more than 10 kcal/mol is much stronger than that for physical adsorption. The heat liberated during chemisorption is in the range of 50-500 kJ/mol. The enthalpy of chemisorption (heat of chemisorption) depends strongly on the surface coverage of adsorbate, largely as a result of adsorbate-adsorbate lateral interactions. It declines with increasing coverage for most systems.
Because, chemisorption observes frequent irreversibility, the chemical nature of the original adsorbate undergoes an alteration in its chemical structure due to strong interactions between reactants. Only a monomolecular layer of adsorbate appears on the adsorbing medium. Chemical bonding between adsorbate and adsorbent is unique owing to the strong attractiveness between the molecules. Hence, the adsorbed molecules cannot freely move on the surface. There is a high degree of specificity and typically a monolayer is formed. The adsorption only happens when there is some possibility of chemical bonding between adsorbent and adsorbate. Likewise, chemisorption increases with an increase in the surface area of the adsorbent. For example, oxygen is adsorbed on metals by virtue of oxide formation and hydrogen is adsorbed by transition metals due to hydride formation.
As chemisorption involves compound formation, the process is seldom reversible. It also happens to be anexothermic process. It is very slow at low temperatures since the reactions have to overcome high activation energy. This means that just like most chemical reactions, chemisorption also increases with the rise of temperature. Accordingly, physisorption of a gas adsorbed at low temperature may be followed by chemisorption with high-affinity molecules at a high temperature once the Ea is supplied. Usually, high pressure is also favourable for chemisorption.
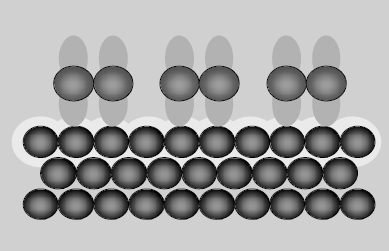
Fig 1: Chemisorption
Table 1:Physisorption vs. Chemisorption at surfaces.
| PHYSISORPTION | CHEMISORPTION |
| WEAK, LONG RANGE BONDING
Van der Waals interactions (e.g. London dispersion, dipole-dipole). |
STRONG, SHORT RANGE BONDING
Chemical bonding involving orbital overlap and charge transfer. |
| NOT SURFACE SPECIFIC
Physisorption is not specific to the type of molecules so it can take place between all molecules on any surface providing the temperature is low enough. |
SURFACE SPECIFIC
E.g. Chemisorption of H2 takes place on specifically transition metals but not on other metals such as gold or mercury. |
| -∆Hads = 5 ….. 35 kJ mol-1 | -∆Hads = 35 ….. 500 kJ mol-1 |
| It is reversible in nature | It is irreversible |
| It usually takes place at low temperature & decreases with increasing temperature | It takes place at a relatively high temperature |
| Non activated with equilibrium achieved relatively quickly. The increasing temperature always reduces surface coverage. | It requires to be activated, in which case equilibrium may be slow and increasing temperature may favour adsorption. |
| No surface reactions. | Surface reactions such as dissociation, reconstruction and catalysis may take place. |
| MULTILAYER ADSORPTION
BET Isotherm used to model adsorption equilibrium. This is a more general, multi-layer model. It assumes that a Langmuir isotherm applies to every layer and that no transmigration happens between layers. It also assumes that there is equal energy of adsorption for each layer except for the first layer. |
MONOLAYER ADSORPTION
Langmuir Isotherm used to model adsorption equilibrium. This model assumes that mono layer coverage takes place with a constant binding energy between surface and adsorbate. |
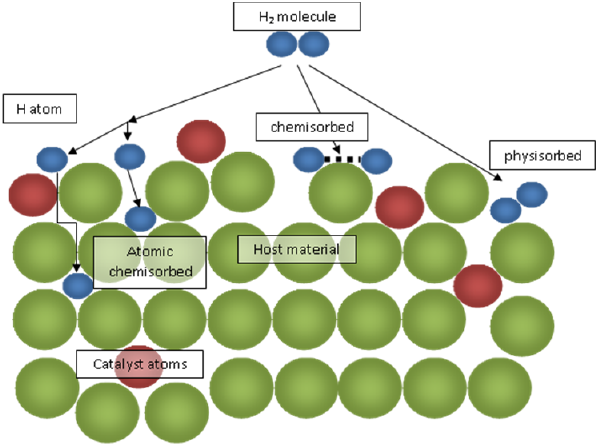
Fig 2: Schematic representation of physisorption and chemisorption for a hydrogen molecule and its interaction with catalyst atoms. |
|