Most of the people feel comfortable with the concept of covalent and ionic bonds, yet they are not sure about what actually hydrogen bonds are, how their formation takes place, and why they are important.
What do you mean by Hydrogen Bond?
Hydrogen bonds refer to the electromagnetic attractions between 2 atoms or the attraction between
The +ve and -ve poles of charged atoms. These bonds fragile (easily broken) and weak, but are responsible for many significant properties of things like DNA and water.
Some of its key points include:
Hydrogen bonds can be formed between atoms in a molecule or between 2 isolated/separate molecules.
The hydrogen bond is much weak than a covalent bond or an ionic bond, but much stronger
Than Van der Waals forces.
A Hydrogen bond plays a crucial role in producing several unique water properties and also a significant role in biochemistry.
A hydrogen bond is an attractive (dipole-dipole) interaction type between the electronegative atoms and the hydrogen atoms which are bonded to other electronegative atoms.
Hydrogen bonds take place between the molecules or in some parts of a single molecule.
A hydrogen bond van is stronger than van der Waals forces, but it is weaker than an ionic or a covalent bond.
It is approximately 1/20 (5

Examples of Hydrogen Bonds
You’ll find Hydrogen bonds in nucleic acids between water molecules and “Base Pairs”. Similar type of bond can also be formed between carbon and hydrogen atoms of different “chloroform molecules”, between nitrogen and hydrogen atoms of adjacent molecules of ammonia, between recurring subunits in polymer like nylon, and between O and H in acetylacetone. A lot of organic molecules are dependent and answerable to hydrogen bonds. Hydrogen bond:
- Arrange polypeptides in secondary structures, like a beta sheet and alpha helix
- Hold 2 strands of DNA together
- Bind each other’s transcription factors
- Assist attaching Transcription Factors (TF) in the direction of DNA
- Support of antigen-antibody interaction or antigen-antibody binding
Hydrogen Bonds: Formation and Properties
Hydrogen bonds are an electromagnetic attraction between the polar molecules where hydrogen element is attached to large atoms like O or N. It is electron sharing, just like in a covalent bond as discussed earlier also.
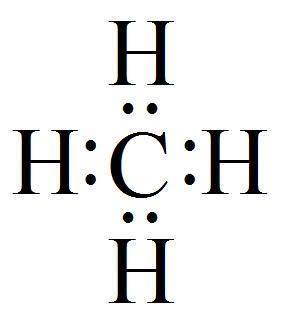
Fig 1.
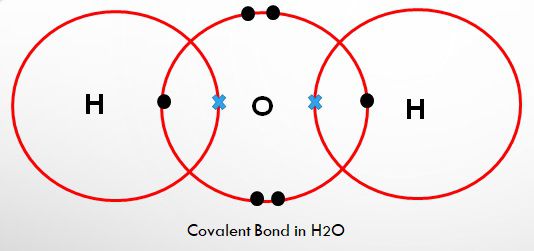
Fig 2.
Like as you can see in figure 2 of the water molecule, how large is O as compared to hydrogen!
Hydrogen atoms are not having the force needed to pull electrons at a distance from larger atoms, so when the hydrogen atoms are inside a covalent bond with a larger atom, the electron lasts a little more time circulating around the larger atom than the smaller hydrogen. It’s positively empty!
As expected, electrons carry negative charge with them, so in whatever direction the electrons will move, the negative charge will follow accordingly.
As a consequence of this uneven or the unequal sharing is the polar atom. It’s an atom which consists of one end which features negative charge and another end features a positive charge.
Now having a polar molecule, it means that it will act similar to a magnet. The positive point of one atom is attracted by the negative point of another atom. So, as a result, there will be a cluster of polar atoms being lined up, throughout.
All these are polar water molecules, lining up end to end, also forming hydrogen bonds. Now, hydrogen bonds are pretty weak. no shared electron have been there, so breaking a hydrogen bond is that much easy. But, these bonds accounts important properties of molecules, within phenomenon is known as water tension.