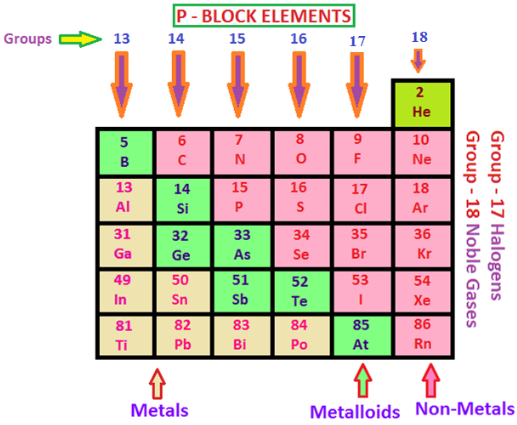
In the P block elements, the last electron enters to the furthest p orbital. In their peripheral shell, there are three to eight electrons. The quantity of p orbitals in the p block elements is three therefore, the maximum number of electrons that can be obliged to this arrangement is six. Consequently, in the periodic table, there are six groups of the p block elements numbering from group 13 to group 18.
Reactivity of the Noble Gasses
The orbitals of the noble gasses are filled by the electrons and breaking their stability is an exceptionally hard thing, whether it is by the addition of electrons or by the removal of electrons. So, low compound reactivity is observed in the case of noble gasses so they are utilized where a nonreactive climate is required, for example in the welding process.
Reactivity of the Halogens
Normally all of the halogens are found in the combined form. Fluorine promptly reacts with any substance that interacts with it. Dynamically, chlorine, bromine, and iodine are less reactive but at the same time, they frame the compounds with the most different elements such as the metals. All the elements of the halogens are the solid oxidizing agents and they oxidize the different substances and get reduced in turn. All the elements of the halogens straightforwardly react with the sodium and produce the sodium halides. They also give reaction with the red phosphorus and produce the halides of phosphorus. They promptly react with the alkali metals and produce the salts. The presence of chlorine, bromine, and iodine can be identified by its treatment with the acidified solution of the silver nitrate.
Reactivity of the Chalcogens (Group VIA Elements)
In this group, the likeness among the elements is most noteworthy. This statement is not valid for the polonium which is different from the other elements due to its radioactivity. The elements of this group react with the electropositive metals and give the X2- particles. At normal temperature and pressure, the oxygen is gas it exists in the two allotropic structures O2 that is the important part of the air and O3 that is ozone and it gradually decays to the oxygen. Ozone ingests the longer wavelengths of the ultraviolet radiation and keeps the harmful rays away from the earth surface thus protecting the life and the environment.
Reactivity of Metalloids
The reactivity of the metalloids greatly depends on the substance with which it reacts so they demonstrate the variable chemical properties. They have low electronegativity, so normally, they are oxidized in the reactions and their oxides are amphoteric.
Reactivity of the Group VA Elements
The elements of this group react with hydrogen and form the trihydrides. Their reactivity is diminished by moving down the group. Upon reaction with halogens, they produce trihalides or pentahalides.
Reactivity of Group IIIA Elements
No element of this group reacts with hydrogen to make the hydrides but all elements react with halogens and produce the trihalides.
Reactivity of Group IVA Elements
Carbon makes solid bonds with other carbon molecules and produces enormous organic compounds. In +2 oxidation state, the lead is reduced but in +4 oxidation state it is a good solid oxidizing agent and, in this state, it forms, the covalent compounds and is firmly bonded to the carbon.