The covalent bond, also known as the molecular bond is the interatomic linkage which is the outcome from electron pair sharing between the two atoms. Or simply you can say that it refers to the chemical bond which includes electron pair sharing between atoms.
The sharing of these electron pairs is called the bonding pairs, or shared pairs, and a stable balance of repulsive and attractive force between the atoms, when there’s sharing of electrons, called as Covalent Bonding.
A covalent bond formation occurs when the bonded atoms consist of lesser total energy compared to broadly divided atoms.
For a lot of molecules, electron sharing allows each and every atom to achieve the corresponding of the complete outermost shell, identical to a stable electronic configuration. As per organic chemistry, covalent bonds are pretty much common as comparative to ionic bonds.
Covalent Bond involves different types of interactions which involves metal-to-metal bonding, π-bonding, σ-bonding, bent bonds, agostic interactions, and 3-center 2-electron bonds.
Molecules that have covalent bonding includes the inorganic elements like water, hydrogen, chlorine, nitrogen, and ammonia (H2O, H2, Cl2, N2, NH3) all at once with entire organic compounds. In molecules’ structural representation, covalent bonds are identified by firm lines which connect atoms pairs. For example, as you can in the diagram below:
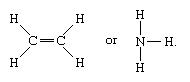
In the diagram, you can see that a single line represents the single bond (i.e. one pair of an electron) while double lines (=) represents a dual bond between the two atoms, which involves two pair of electrons; and triple lines (≡) indicates the triple bond. For eg. (C≡O) is a triple bond.
The Single bond has one sigma bond represented by (σ), a double bond is represented by a single (π) bond, and the triple bond is indicated by a single (σ) sigma and two pie π bonds.
The concept that the sharing of two electrons can be done between the 2 atoms and act as a link between the electrons and atoms which was initially introduced in 1916 by renowned American chemist named, G.N Lewis.
He explained that some specific atoms combine with another to form the electronic structure of an equivalent “noble-gas atom”.
Covalent bonds happen to be directional, which means bonded atoms favor particular orientations comparative to another one, this in order provides definite shapes to molecules, just like the angular structure (bent structure) of water (H2O) molecule.
A covalent bond between similar atom, for eg. in H2, is non-polar (similar or identical atoms are non-polar) – that is, it’s electrically uniform. On the other hand, dissimilar or unlike atoms, are polar, which means a single atom is a little negatively charged while the other is marginally positively charged. The incomplete ionic nature about covalent bonds accelerates with an electronegativity difference between the two respective atoms.
Also, there are 3 types of covalent bonds
- Polar Covalent
- Non-polar Covalent
- Co-ordinate Covalent
Polar Covalent: The formation of Polar Covalent takes place when non-metals have electronegativity difference.
Non-Polar Covalent: The formation of Non-Polar Covalent takes place between atoms having similar electro negativities..
Co-ordinate Covalent: The formation of Co-ordinate Covalent bond takes place between ligands and transition metals. The atoms or compounds share their electron pairs of non-bonding with the vacant D orbit of the transition metal.