Ozone processes tend to be based on the effects of various indirect and direct reaction mechanisms. This is something significant when it comes to the disintegration of ozone into water, considering the OH radicals. These radicals are supposed to be short-lived compounds and possess much stronger oxidization abilities in comparison to ozone.
Ozone is present naturally in the atmosphere. Containing three oxygen atoms, an Ozone molecule is represented as O3 . It was known to be discovered in the 1800s during several laboratory experiments.
Stratospheric Ozone
Stratospheric ozone is known to be formed naturally by chemical reactions that generally involve solar ultraviolet radiations i.e. sunlight and oxygen molecules present around and constitute about 21 percent of the atmospheric gases. During the first step of the procedure the ultraviolet radiations break each oxygen molecule (O2) to attain two oxygen atoms. These highly reactive atoms then tend to combine with oxygen molecules (O2) for the formation of an ozone molecule (O3). These reactions continue to take place in the presence of solar ultraviolet radiations.
Tropospheric Ozone
Ozone is known to be produced by chemical reactions that involve naturally existing gases and other gases that are obtained from pollution sources. These reactions initially involve nitrogen oxide gases and hydro carbons, in fact, ozone itself. All of this additionally requires sunlight for completion. For tropospheric ozone production, fossil fuel combustion is known to be the primary source of pollutant gases. The ozone that is produced near the surface, does not contribute to tropospheric ozone formation much significantly. The share of stratospheric ozone is way too less and the transfer of atmospheric air to the stratosphere isn’t really helpful for the reactions.
Direct Ozone Reactions
According to its structure, ozone is capable of acting like a 3-dipole, a neucleophilic agent and an electrophilic agent during the reactions. In solutions that consist of organic pollutants, three types of reactions are likely to occur:
Cyclo Addition
With effect to its dipolar structure, an ozone molecule can get through a 1-3 dipolar cyclo addition that involves saturized compounds (double or triple bonds). As a result a compound called ozonide is formed.
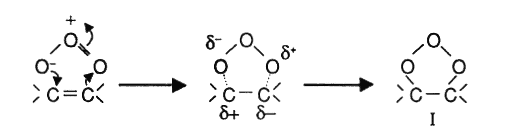
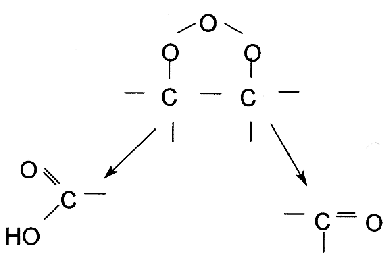
In protonic solutions like water, the ozonide disintegrates into an alehyde that would either be a keton or a zwitter ion.
Electrophilic Reactions
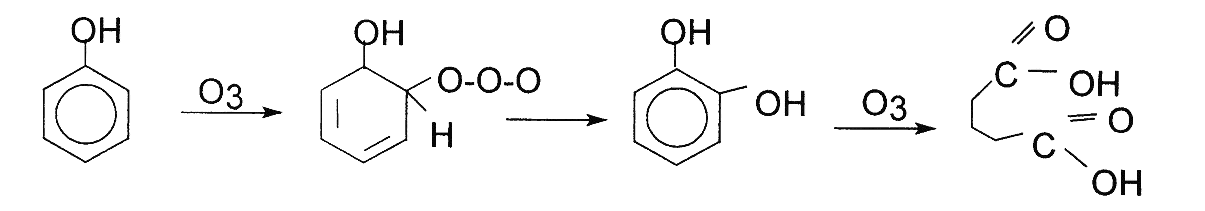
Electrophilic reactions tend to occur in molecular solutions that possess higher electronic density. Also, these are generally solutions that have significantly high level of aromatic compounds. Aromatic compounds that tend to be substituted by electron donors like NH2 and OH carry higher electronic density on carbon compounds in the para and ortho position. Aromatic compounds therefore react actively with Ozone.
Nucleophilic Reactions
Nucleophilic reactions generally occur where there is a shortage of electrons. This particularly happens with carbon compounds that contain the electron retreating groups like –NO2 and – COOH. The reaction speed for such groups considerably lower.
For most of the inorganic compounds like water, the reaction speed tends to be relatively higher. The transfer of the oxygen atom of ozone to the inorganic compounds generally determines the oxidization mechanism. For ionized and disassociated inorganic compounds too the reaction speed tends to be higher. Most of the inorganic compounds react faster and completely with ozone.