Chemical Thermodynamics: Intensive properties
Intensive properties are independent of the amount of mass of a system and are a local physical property. Therefore, it is a bulk property. If a quantity of substance in a given state is divided into two parts with intensive and extensive properties, each part will have equal number of these components.
Examples of intensive properties include:
- chemical potential
- concentration
- density (or specific gravity)
- ductility
- elasticity
- electrical resistivity
- hardness
- magnetic field
- magnetization
- malleability
- melting point and boiling point
- molar absorptivity
- pressure
- specific energy
- specific heat capacity
- specific volume
- spectral absorption maxima (in solution)
- temperature
- viscosity
Mechanical work and heat can not be defined as properties of a system. They characterize the mode of energy exchange between the system and the surroundings. Likewise, they depend on the path or specific transition of the process.
The ratio of any extensive property of a system to that of the mass of the system is called an average specific value of that property.Some intensive variables have no extensive counterpart, such as pressure or temperature. Intensive properties can be functions of time and position, however extensive properties vary at most with time.
In a thermodynamic analysis, it is easier to analyse intensive properties since we can eliminate the mass from the analysis. As the mass and volume are completely assosiciated to each other under standard situations, we outcome a new property called the particular volume which is same as to the volume divided by the mass. The “specific” of specific volume essentially means “divided by mass”.
For example, the density of water is an intensive property and it can be derived from the mass of water (an extensive property) divided by the volume (another extensive property). Also, heat capacity, an extensive property of a system can be derived from heat capacity, Cp, and the mass of the system. Dividing the extensive properties gives the specific heat capacity, cp, which is an intensive property. Specific properties as shown in the table below present a means of recording material data in a manner that is independent of size or mass. They are very useful for making comparisons about one attribute while cancelling out the effect of variations in another attribute.
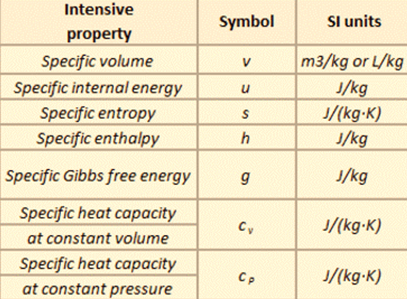
Figure 1: Examples of intensive properties
Temperature characterizes the thermal state of a body. It is common knowledge that heat can flow spontaneously only from bodies at a higher temperature to bodies at a lower temperature. Hence, the temperature of bodies determines the direction of the possible spontaneous heat exchange between these bodies. Likewise, the temperature is an intensive property. So if one block of ice is at −10◦ C then adding another identical block does not make the temperature −20◦ C, but it does mean that melting the two blocks of ice can be predicted to use twice as much energy.
According to the state postulate, for a simple thermodynamic system, only two independent intensive variables are needed to specify the state of a system. Other intensive properties can be derived from any two known values.
Some intensive properties, such as viscosity, are empirical macroscopic quantities and are not relevant to extremely small systems.