Ionic solids refers to the constituent particles which are ions. These solids are formed as a result of 3-D arrangement of anions and cations. The forces acting between anions and cations are electrostatic forces or coulomb’s forces of attraction. These forces exhibit strong nature.
Eg: In NaCl compound, Na+ is called cation and Cl- as the anion). Also, (CaF2), Calcium Fluoride are the examples of Ionic Solids.
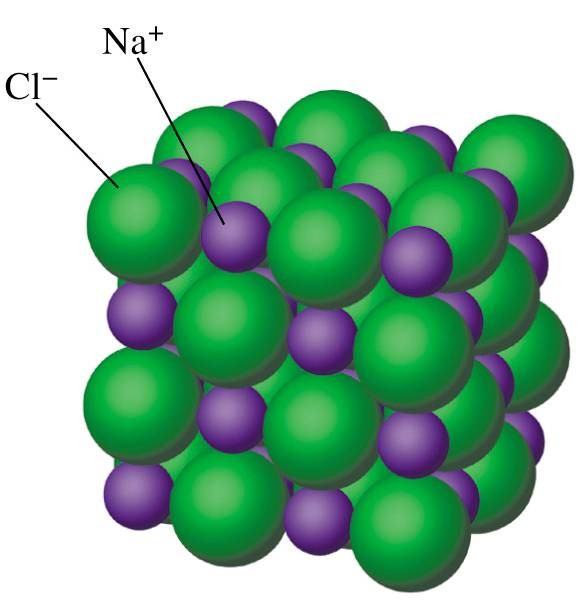
The above image is of NaCl structure
Ionic solids represent the following properties, which are:
- Ionic Solids are brittle or fragile, and hard in nature
- They have low volatility because of the presence of high vaporization and also high enthalpy of fusion.
- They even have high melting points
- In solid state, they are poor conductors of electricity, but when these Ionic solids are dissolved or melted, they are good conductors of electricity.
- This solid type is soluble in polar solvents such as water, ammonia, sulphuric acid
As mentioned earlier also, Ionic solids consist of negatively charged anions and positively charged cations. These solids are composed of charged ions which are opposite in nature.
Also, ionic solids consist of positive and negative charged ions which are together held by electrostatic or coulomb’s forces. There’s a simple composition of ions in a NaCl compound or ionic solids can be the composition of polyatomic ions as you can see in NH4NO3 (Ammonium Nitrate) formed with ammonium (NH4+) and nitrate ions (NO3-).
As shown in Equation 1, The product of two charged particles i.e. Q1 and Q2 is directly proportional to the electrostatic energy and this electrostatic energy is inversely proportional to the distance between the particles, denoted by ‘r’
Electrostatic energy∝Q1Q2/r
where,
Q1 and Q2 = Electrical charges present on charged particles named 1 and 2,
r = distance between charged particles.
When the charged particles (Q1 and Q2) are positive in nature, having close similarity to the charge acting on cations, and then there’s repulsion between cations, and there’s positive electrostatic energy.
And when the particles Q1 and Q2 are negatively charged, relative to charge present on anions, then there’s repulsion between anions, and there’s again positive electrostatic energy.
.
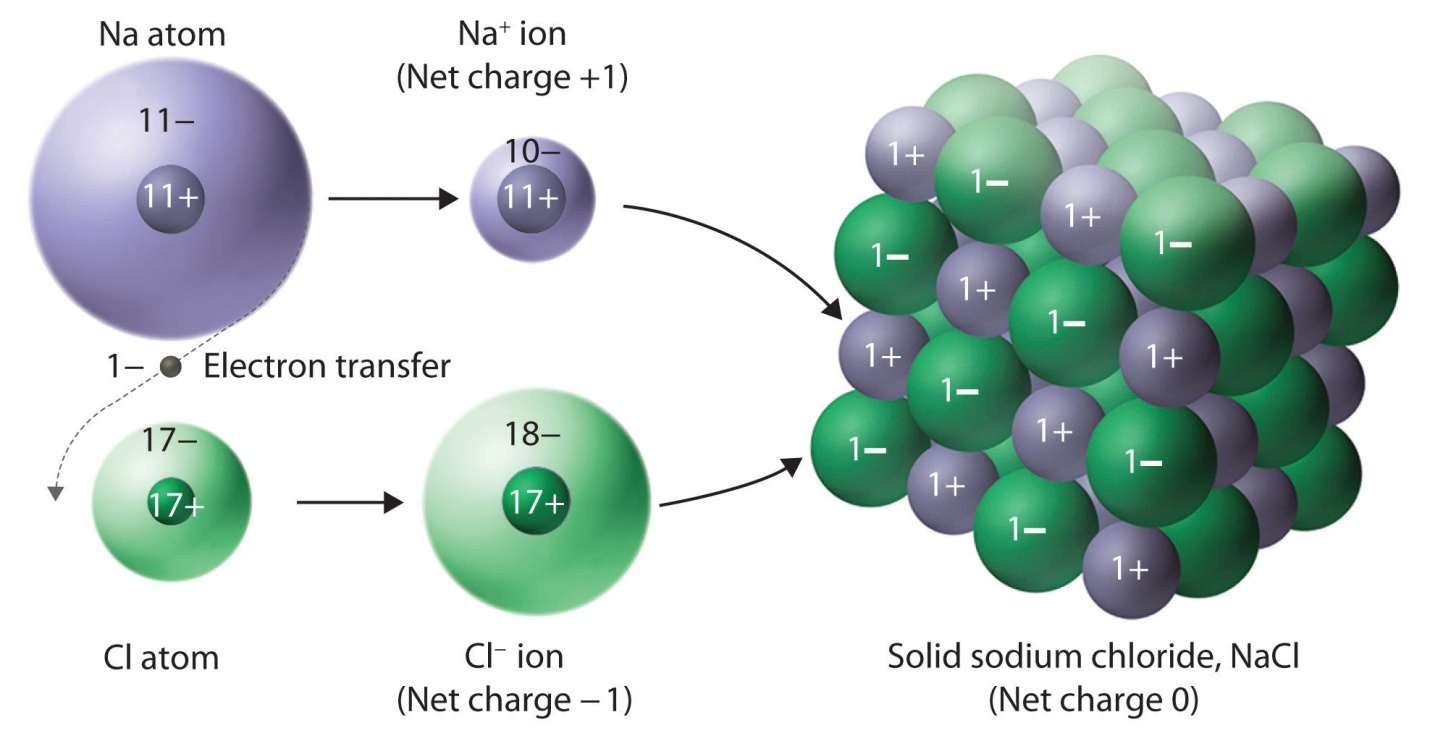
Fig: The ionic compound NaCl forms when electrons from sodium atoms are transferred to chlorine atoms. The resulting Na+ and Cl− ions form a three-dimensional solid that is held together by attractive electrostatic interactions.
Lattice Energy
The lattice energy is directly proportional ionic charges’s product and inversely proportional to the total of ions’ radii.
Because mostly the lattice energies of ionic compounds are high, therefore, the ions don’t separate themselves so easily from their crystalline structure switched into the gaseous state.
Generally, Ionic Solids don’t switch to gaseous state from Solid state under normal temperature conditions. However, they can be melted by exerting sufficient thermal energy to interfere the crystalline lattice. Hence, the more is the lattice energy, the more is melting point.
Energy which is necessary to separate an ionic crystal when it is in dissolved form, then it comes with interaction of ions in (solid) crystal state with solvent molecules.
Ionic solids’ lattice energy is an ionic solid that relies on dissolving in a solvent. Thus, the less is the lattice energy, the greater the ionic solids’ quantity is which can be dissolved in any solvent quantity.