Surface Chemistry: Lyophobic, multimolecular and macromolecular colloids
Two phases are involved in a colloidal system
- Dispersed phase – solid, liquid, gas
- Dispersion medium (continuous phase) — solid, liquid, gas
General types of colloidal system:
- Lyophobic colloids are solvent hating colloids(Lyo means solvent and phobic means hating). There is minimal or no affinity between particles of the dispersed phase and the dispersion medium. For instance, metals and their sulphides simply mix with dispersion medium and don’t form colloids. This type of colloidsneedspecial techniques for their preparation and stabilizing to preserve them and the mixture is irreversible. Once the mixture precipitates, it cannot be returned to its previous state.A lyophobic sol is thermodynamically unstable.The particles in such system are stabilized only by the presence of electrical charges on their surfaces. The addition of a small amount of electrolyte to a lyophobic sol tends to stabilize the system by imparting a charge to the particles. Examples include colloidal sols of gold,silver, Fe(OH)3, As2S3, etc.
- Amphiphiles or associated colloids are surface active agents characterized by having a hydrophilic head and a lipophilic tail.When present in a liquid at low concentration the amphiphiles exist distinctly and are present in a sub-colloidal size range.
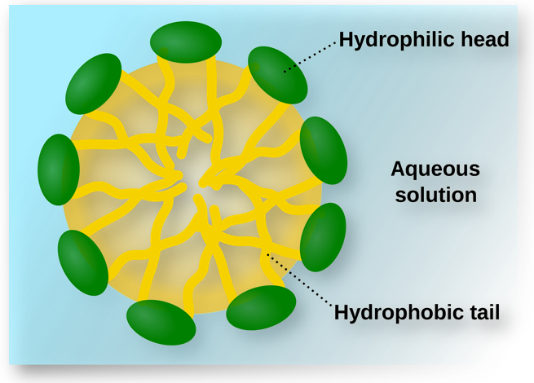
Fig : Associated colloid
- Multimolecular colloids consist of aggregates of many atoms or smaller molecules (diameter< 1 nm). These colloids usually have lyophobic character. Eg: gold and sulphur sols. Small molecules (or atoms) are held together by weak Van der Waal interactions. Usually, these colloids have a lyophobic nature. This means these colloids have less or no attraction forces with the dispersion medium.
- In the macromolecular colloids, the molecules have sizes and dimensions comparable to colloidal particles (100 nm). These colloids usually resemble true solutions. For example, proteins, starch, cellulose.Most of the lyophilic colloids fall into this category of colloids.
- Aerosol: solid or liquid disperses in gas
- Emulsion: liquid inliquid phase
- Foam: gas disperses in liquid (or solid)
- Gel: consists of two inter-penetrating networks, and hard to specify which is dispersed and which is a continuous phase.
| Multimolecular colloids | Macromolecular colloids | Associated colloids |
| Formed by aggregation of a large number of atoms or molecules with diameters less than 1 nm | Formed by individual molecules that are large enough in colloidal range to be considered as a colloid. | Formed by aggregation of a large number of ions in concentrated solution |
| Lyophilic colloid | Lyophobic colloid | Both lyophilic and lyophobic in nature |
| Molecular weight is intermediate | High molecular weight | High molecular weight |
| When the compound is added to a dispersion medium, small molecules form aggregates with dimensions in the colloidal range. | When the compound is added to a dispersion medium, compound separates into individual molecules which have their dimension in colloidal range. | When the compound is added to a dispersion medium, it chooses to be in both states: aggregates and individual molecules. |
| Held by weak van der Waals’ forces | Held by stronger van der Waals’ forces due to the long chains | van der Waals’ forces increase with the increase in concentration |