Mole concept is a method to express amount of substance. It is widely used when dealing with particles at atomic or molecular level. Its symbol is written as mol. It is defined as the amount of substance that contains exactly 6.02214076×1023 particles of a given substance.
To understand the concept let’s take an example of the reaction of hydrogen and oxygen to form water: 2H2 + O2 → 2H2O.
This reaction indicates:
- 2 Molecules of water are formed by combining 2 molecules of hydrogen with 1 molecule of oxygen.
- 4 u of hydrogen molecules when combined with 32 u of oxygen molecules forms 36 u of water molecules
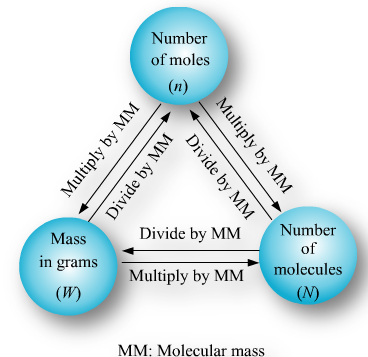
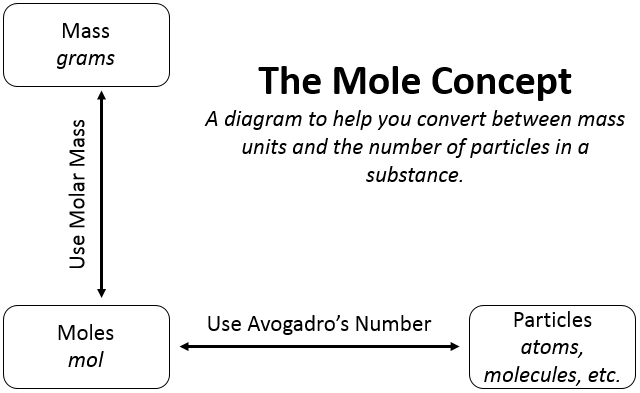
It can be inferred from the above equation that the quantity of a substance can be characterised by its mass or the number of molecules. But, a chemical reaction equation directly shows the number of atoms or molecules taking part in the reaction. Therefore, it is always convenient to refer to the quantity of any substance in terms of the number of its molecules or atoms, rather than their masses. So, a new unit mole was introduced.
One mole of any atom or molecule is that quantity in number having a mass equal to its atomic or molecular mass in grams. The number of particles present in 1 mole of any substance is fixed, with a value of 6.022 × 10^23. This is an experimentally obtained value. This number is termed as Avogadro Constant and is represented by symbol N0. It is named in honour of the Italian scientist Avogadro.
1 mole = 6.02214076×1023 in number for any substance.
1 mole of a particular substance also has fixed mass and is equal to its relative atomic or molecular mass in grams. The mass of one atom of an element in atomic mass units is given by the atomic mass of that element. To get the mass of 1 mole of atom of that element, which is also called its molar mass, we have to take the same numerical value but change the units from ‘u’ to ‘g’. Molar mass of atoms is also called as gram atomic mass. For example, atomic mass of hydrogen=1u. Also the gram atomic mass of hydrogen = 1 g. So 1 u hydrogen has only 1 atom of hydrogen and 1 g hydrogen has 1 mole atoms, that is, 6.022×1023 atoms of hydrogen.
For carrying out various reactions chemists need the number of atoms and, and for this they need to relate the mass in grams to the number. It is done as follows:
1 mole = 6.022×1023 which is relative mass in grams.
Thus, a mole is the chemist’s counting unit. Wilhelm Ostwald around 1896 introduced the word mole. He derived the term from the Latin word moles meaning a ‘heap’ or ‘pile’. A substance may also be considered as a pile of atoms or molecules. The unit mole was accepted in 1967 to provide a simple way of reporting the massive heap of atoms and molecules in a sample.