p-Block Elements: Group 16
Trends in physical and chemical properties
In group 16 there are two non-metals: Oxygen and Sulphur, Two metalloids: selenium and tellurium and two radioactive metals: polonium and livermorium. All of these elements exhibit allotropy. The melting and boiling points of the elements increase with an increase in atomic number down the group. Notice that there is a large difference in mp and bp from oxygen to Sulphur owing to the basis of their atomicity; oxygen exists as a diatomic molecule (O2) whereas sulphur exists as a polyatomic molecule (S8). The mp and bp decrease from Te to Po because of the ionic bonds present in Po.
Atomic and Ionic Radii:
With an increasing number of shells, atomic and ionic radii increase from top to bottom in the group. The size of the oxygen atom is exceptionally small compared to other elements which cause the valence electrons to stay closer to the nucleus and preventing their loss.
Electronegativity:
After fluorine, oxygen has the highest electronegativity value. Within the group, electronegativity decreases as the atomic number increases. Likewise, the metallic character
increases from oxygen to polonium.
Ionisation enthalpy:
Ionisation enthalpy of elements of group 16 is lower than group 15 due to half-filled p-orbitals in group 15 which are more stable. But ionization enthalpy decreases down the group due to increasing shielding effect from the nucleus.
Electron gain enthalpy:
The compact nature of the oxygen atom allows it to have less negative electron gain enthalpy than sulphur. There is a rise in electron gain enthalpy at sulphur and then the value again becomes less negative to polonium because of the valence electrons further away from the nucleus. Hence it becomes easier for them to gain an electron rather than loosing.
Reactivity with hydrogen:
All group 16 elements combine with hydrogen to form hydrides. Acidic nature and bent shape decrease from water. The H-E bond length increases as the bond dissociation enthalpy decrease down the group. Thermal stability and reducing character also decreases because the H-E bond length increases down the group.
Acidic nature: H2O < H2S < H2Se < H2Te
Thermal stability: H2O < H2S < H2Se < H2Te < H2Po
Reducing character: H2O < H2S < H2Se < H2Te < H2Po
Reactivity with oxygen: EO2 and EO3
The reducing character of dioxides decreases down the group. Oxygen has a strong +ve field which attracts the hydroxyl group and removal of H+ becomes easy. The acidity of oxides also decreases down the group. SO2 is a gas of discrete units whereas SeO2 is solid that has a chain polymeric structure.
Reactivity with halogens: EX2 EX4 and EX6
The stability of halides of group 16 elements decreases in the order F- > Cl- > Br- > I- . As the atomic size increases the E-X bond length also increases. Fluorides in EX6 state are the most stable because of steric nature. H2O is a liquid while H2S is a gas due to strong hydrogen bonding present in water and the compact nature of oxygen.
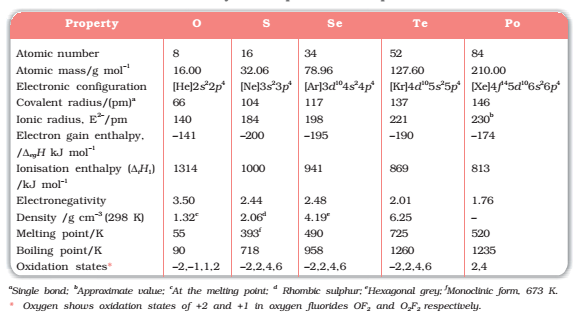
Fig1 :Some physical and chemical properties of group 16 elements.