In 1932 the neutron was discovered by English Physicist, James Chadwick. At that time, it was known that atom has a nucleus which is positively charged and is surrounded by the electrons and negative charge is enough to make an atom electrically neutral. About 12 years earlier a postulate was stimulated by Ernest Rutherford who was a pioneer in the atomic structure. He stated that a neutral particle does exist with the approximate mass of a proton and it could be resulted due to capturing of an electron by the proton. This postulate stimulated the research for this particle. However, the situation was complicated due to the electrical neutrality of the atom because, at that time, all the experimental techniques had measured the charged particles only.
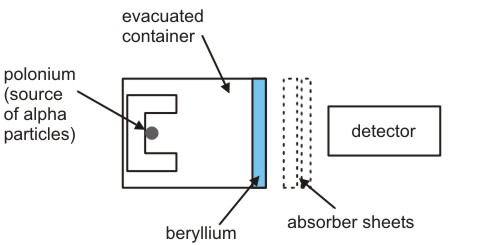
The results presented by Ernest Rutherford arose the disparity between the atomic mass and an atomic number of an element. An initial step was taken by German Physicist and his student in 1928. They conducted an experiment and bombarded beryllium along with the alpha particles which were emitted from the polonium and they found electrically neutral and penetrating radiation and it was interpreted by them as gamma photons of high energy.
A task was assigned to James Chadwick to track down the evidence of neutron. Then Chadwick conducted experiments in England in the Cavendish Laboratory of Cambridge. In his experiment hydrogen atoms were bombarded in paraffin with beryllium emissions. Additionally, nitrogen, helium, and some other elements were used by him as a target. He compared the energies of recoiled charged particles which were obtained from different targets and was able to prove that a neutral component is associated with beryllium emissions and approximately its mass is equal to the mass of a proton. By conducting the experiments, he determined that neutron exists and its mass is more than a proton and the mass is greater as 0.1
Quickly his findings were accepted and then Werner Heisenberg showed that neutron could not be the result of the pairing of electron-proton. Instead, neutron is a unique particle and it was considered as 3rd piece of the atom after electron and proton. Based on the results of Chadwick’s experiment, Heisenberg proposed the model of proton-neutron for the nucleus.
By this idea, the picture of the atom was dramatically changed and this concept accelerated the discoveries in the discipline of atomic physics. Soon it was revealed that neutrons can be used as an ideal bullet for bombarding the other nuclei. Due to its unique property that it was not repelled by the similar charged particles (like electron and proton do) it could directly smash to the nucleus.
Later on, neutrons were bombarded to uranium atom for splitting the nucleus and to obtain the huge amount of energy as predicted by Einstein’s equation.
E=mc2