d and f Block Elements
Electronic configuration
The general electronic configuration for first and second transition series can be written as ns2(n − 1)dx
- First = [Ar]4s23dx; second = [Kr]5s24dx
For third and fourth transition series, the general electronic configuration isns2(n − 2)f14(n − 1)dx
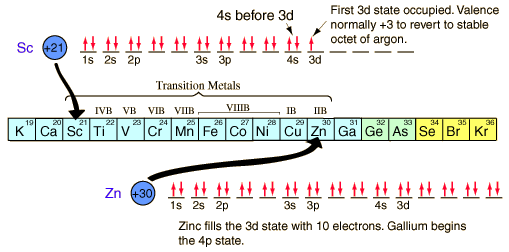
Some elements deviate from the general pattern by “promoting” one or more s electrons into the d orbital to complete the subshell. Because of sublevel splitting, the 4s sublevel is lower in energy than the 3d; therefore, the 4s fills before the 3d. However, the difference in energy is not large.The electron configuration of d block elements across the 1st row of the d block (Sc to Zn) is given in the table below. Each element in this row has 1 more electron and 1 more proton where each “additional” electron enters the 3d sub-shell and the electronic configuration of argon is Ar: 1s22s22p63s23p6
| Element | Core electrons | 3d electrons | 4s electrons |
| Sc | [Ar] | 3d1 | 4s2 |
| Ti | [Ar] | 3d2 | 4s2 |
| V | [Ar] | 3d3 | 4s2 |
| Cr | [Ar] | 3d5 | 4s1 |
| Mn | [Ar] | 3d5 | 4s2 |
| Fe | [Ar] | 3d6 | 4s2 |
| Co | [Ar] | 3d7 | 4s2 |
| Ni | [Ar] | 3d8 | 4s2 |
| Cu | [Ar] | 3d10 | 4s1 |
| Zn | [Ar] | 3d10 | 4s2 |
In the electronic configuration, the 4s orbital is filled before the 3d orbitals. This is because before filling orbitals, 4s orbitals have lower energy than 3d orbitals. The reversed order of the 3d and 4s orbitals only applies to build the atom up in the first place.
Scandium has the electronic structure [Ar] 3d14s2. In ionic form, it loses the 3 outer electrons to make an argon structure. However, the Sc3+ ion does not have d electrons and so doesn’t meet the definition of transition metal. Similarly, Zinc has the electronic structure [Ar] 3d104s2. Loss of two 4s electrons gives a 2+ ion with the electronic structure [Ar] 3d10. This zinc ion has full d orbitals and doesn’t meet the definition of transition metals either.
Copper, [Ar] 3d104s1,can form two ions. The Cu+ ion: [Ar] 3d10 while Cu2+ ion :[Ar] 3d9, this means that Copper is definitely a transition metal because the Cu2+ ion has an incomplete d orbitals. Cr and Cu are electron configuration exceptions because they prefer half-filled and filled d-orbitals for stability. At Cr the orbital energies such that putting one e- into each 3d and 4s orbital gives lower energy than having 2 e- in the 4s orbital. At Cu, putting 2 e- into the 4s orbital would give higher energy than filling the 3d orbitals
To write the electronic structure for Co2+:
The 2+ ion is formed by loosing two 4s electrons.
To write the electronic structure for V3+:
The 4s electrons are lost first and then 3d electrons are lost.
Some of the transition metals have irregular electron configurations in which the ns only partially fills before the (n − 1)d or doesn’t fill at all. Therefore, their electron configuration must be found experimentally.
The transition elements show more than one oxidation state and these states are related to patterns in successive ionization energies. The first-row d-block elements form ions as they lose the 4s electrons first in order to make the 2+ ions. To make ions of oxidation state higher than 2+, they start losing the 3d electrons. Because the 3d and 4s orbitals are close in energy, the electrons can be removed without a huge jump in energy as you would see from the s and p orbitals.
Consider the 2 examples:
Ca: 1s22s22p63s23p64s2
Ti: 1s22s22p63s23p63d24s2
Calcium will lose the 4s2 electrons and then it would take a huge amount of energy to pull off the electrons in the 3p orbital. Titanium will lose the 4s2 electrons to make Ti+2, then one of the 3d electrons to make Ti+3, then the other 3d electron to make Ti+4. It refrains from making a +5 ion because it takes too much energy to pull off the p electrons.