The Amines are compounds that are derived from Ammonia group. The ammonia group has one nitrogen atom bonded with four hydrogen atoms respectively. The Amines are derived from the Ammonia by replacing one or more than one hydrogen atom connected to nitrogen. The group bonded is either an Alkyl group or any Aryl group respectively.
Depending upon the number of hydrogen atoms replaced the Amines are categorized. The type of Amines depending upon the number of hydrogen atoms replaced is 1˚,2˚ ,3˚. In the one degree amines only one hydrogen atom is replaced with an alkyl or an aryl group. In the two degree amine two hydrogen atoms are replaced by different alkyls and aryls. Whereas in the three degree amines three hydrogen atoms are replaced respectively.
IUPAC Nomenclature of Amines:
As such the nomenclature of amines does not follow any specific rule. Depending upon the type of groups and the degree of amine, the nomenclature of amine is done. In a hydrocarbon chain in which the amine is attached is numbered. This numbering of carbon atoms is done in accordance to the preferences of other functional groups if present in the chain. If only amine group is present, then number the carbons in such a way that that the amine group gets the least number. Then accordingly the naming of the compound is done in accord with IUPAC norms.
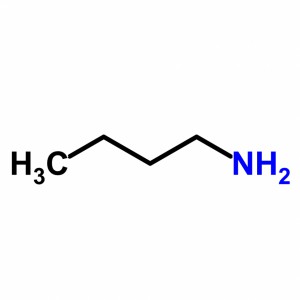
In this compound, the amine group is attached to the first carbon. Now, we can number the carbon atom of the compound in such a manner that the Amino group gets least number. Therefore, upon numbering the atoms we get the amino atom located at 1 position. The rest of the compound is butane. Hence, the name of the compound will be 1-Amino butane, according to the nomenclature. Also, when there is 1˚ Amine attached, it is termed as Amino.
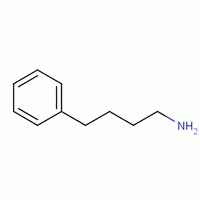
In this compound we can see that along with the Amino group, there is also the phenyl group attached to the hydrocarbon. Now, we may do the numbering of the carbon atoms in the hydrocarbon. We know, in accordance to the nomenclature; the amine will get more preference than the phenyl group. Hence, the numbering of the carbon will be done from the amino side. Hence, we will give one number to Amino and the Phenyl group will get number four. Therefore, the compound name will be 1-Amino 4-Phenyl butane.