p-Block Elements: Coordination compounds
IUPAC nomenclature of mononuclear coordination compounds
- The cation comes first, then the anion(s). These ions are not in the coordination sphere.
- diamine silver(I) chloride [Ag(NH3)2]Cl
- potassium hexacyanoferrate(III) K3[Fe(CN)6]
- Inner Sphere Complex Ion is enclosed by using brackets. Within this sphere, the ligands are named first and then the metal, however, in writing formulas, the metal ion is written first.
- Ligands are named before the metal
- Metal is written first in the formula
- A space only between cation and anion
- No capitalization is needed
- tetraamminecopper(II) sulfate [Cu(NH3)4]SO4
- hexaamminecobalt(III) chloride [Co(NH3)6]Cl3
- Prefixes denote the number of each type of ligand. Special prefixes and parentheses denoted when the ligand is already containing a prefix.Within this sphere, the ligands should be named before the name of the metal, but in formulas, the metal ion is written first as was the case with sphere.
2 Di Bisnonakis 3 Tri Tris 4 Tetra Tetrakis 5 Penta Pentakis 6 Hexa Hexakis 7 Hepta Heptakis 8 Octa Octakis 9 Nona Nonakis 10 deca decakis - dichlorobis(ethylenediamine)cobalt(III) fluoride [Co(en)2Cl2]F
- tris(bipyridine)iron(II) chloride [Fe(bipy)3]Cl2
- Ligands are named in alphabetical order and prefixes are not counted.
- tetraamminedichlorocobalt(III) [Co(NH3)4Cl2]+
- amminebromochloromethylamineplatinum(II) [Pt(NH3)BrCl(CH3NH2)]
- Ligand name alterations to consider:
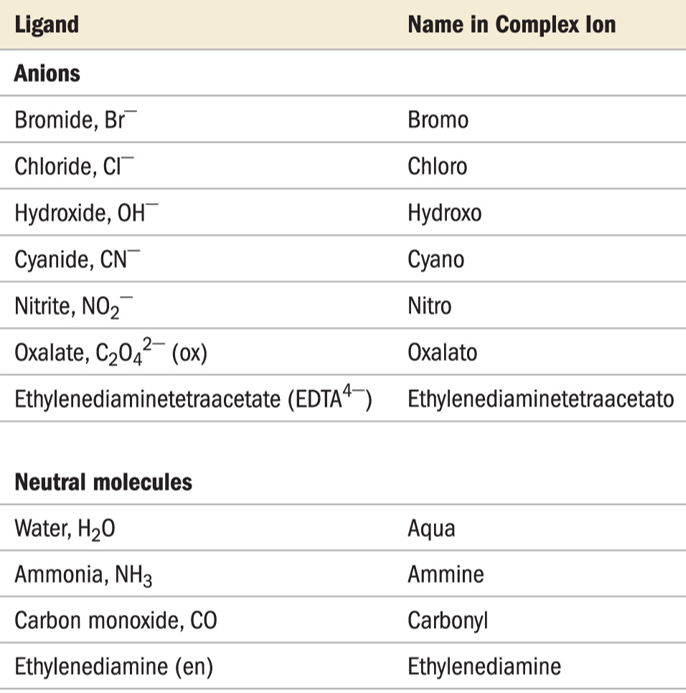
- Anionic ligands are given an -o suffix: chloro, fluoro, oxo, sulfato
- Neutral ligands keep their name: methylamine, bipyridine
- Water becomes aqua
- NH3 becomes ammine to keep separate from alkylamines
- On of the two systems can be followed for showing charge or oxidation state of metal ion
- Stock system: oxidation state in Roman Numerals in parenthesis after the metal ion name. This is the most common method.
- Ewing-Basset system: charge of the total complex ion is placed in parenthesis after the name of the metal ion.
- Both systems add –ate to the metal name if the complex ion has an overall (-) charge
- [Pt(NH3)4]2+ = tetraamineplatinum(II) or (2+)
- [PtCl4]2- =tetrachloroplatinate(II) or (2-)
- [PtCl6]2- =hexachloroplatinate(IV) or (2-)
- Isomer designations are placed before the rest of the name and in italics
- cis-diamminedichloroplatinum(II)
- trans-diamminedichloroplatinum(II)
- Bridging ligands are denoted with the prefix μ
- [(NH3)4Co(OH)(NH2)Co(NH3)4]4+ = μ-amido-μ-hydroxobis(tetraaminecobalt(III))
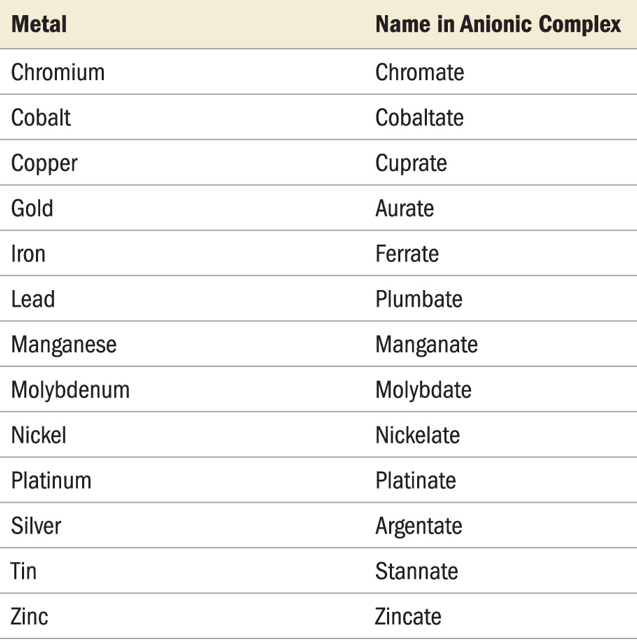
- Negatively charged complexes of metals are shown in their Latin names:
Fe = ferrate Ag = argenate Sb = stibate Au = auratePb = plumbate Sn = stannate Au = aurate
Exception: Hg still called mercurate (Latin name: hydrargyrum)
Examples of Naming Coordination Compounds:
| Name [Cr(H2O)5Cl]Cl2 | Name K3[Fe(CN)6] | |
| Identify the cation and anion. Add the name of the simple ion. | [Cr(H2O)5Cl]2+ is a complex cation;
Cl− is chloride. |
K+ is potassium;
[Fe(CN)6]3− is a complex ion. |
| Give each ligand a name and list them in alphabetical order. | H2O is aqua;
Cl− is chloro. |
CN− is cyano. |
| Name the metal ion. | Cr3+ is chromium(III). | Fe3+ is ferrate(III) due to the complex ion beings anionic. |
| Name the complex ion by adding prefixes to indicate the number of each ligand followed by the name of the metal ion. | [Cr(H2O)5Cl]2+ is pentaquochloro-chromium(III). | [Fe(CN)6]3− is hexacyanoferrate(III). |
| Name the compound by writing the name of the cation before the anion. The only space is between ion names. | [Cr(H2O)5Cl]Cl2 is pentaquochloro-chromium(III) chloride. | K3[Fe(CN)6] is potassium hexacyanoferrate(III). |