p-Block Elements: Group 16
Oxoacids of sulphur
Sulphur oxoacids are compounds that comprise of Sulphur, hydrogen and oxygen. Sulphur forms a number of oxoacids such as H2SO2, H2SO3, H2S2O3, H2S2O4,H2S2O5, H2SxO6 (x = 1 to 5), H2SO4, H2S2O7, H2SO5, H2S2O8. Multi oxide acids are unstable and cannot be isolated. They are produced in a ratio of an aqueous solution or in the form of their salts.
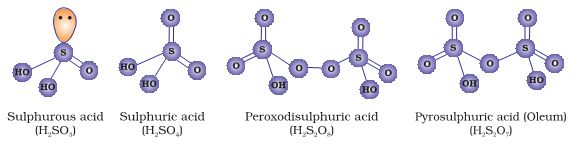
The sulphurous acid series groups di or pyro-sulphurous acid and dithionous acid. The sulphuric acid, thiosulfuric acid and pyrosulphuric acid are grouped in sulphuric acid series. Dithionic and polythionic acid are a part of thionic acid series. Lastly, peroxomonosulphuric acid and peroxodisulphuric acid form peroxo acid series.
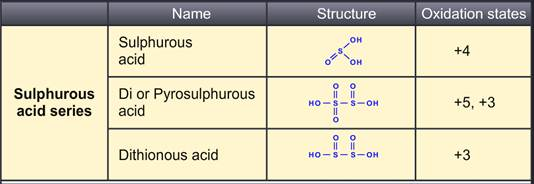
Sulphurous acid is prepared by dissolving SO2 in water. A saturated solution as 3°C can also give crystals of sulphurous acid (H2SO3.6H2O). The SO3 ion has a pyramidal structure with three oxygen molecules in triangular. Lone pair distorts the tetrahedral structure to form pyramidal structure. It is a strong reducing agent with bleaching properties. Di-Pyrosulphurus acid does not occur in the free state. Dithinious acid is prepared by reducing sulphurous acid containing sulphites with amalgamous zinc or zinc dust.
2S2O42- + H2O S2O32- + 2HSO3–
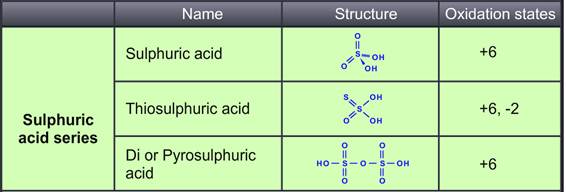
Sulphuric acid is prepared by the contact process. The Sulphur atom is bonded to two oxygen atoms with double bonds and two hydroxyl groups (OH) through single bonds. Hence it is diprotic in nature and highly corrosive strong mineral acid. The molecular formula is H₂SO₄.It is pungent and colourless to a slightly yellow viscous liquid that is soluble in water at all concentrations. Thiosulphuric acid is prepared by reacting Sulphur trioxide with hydrogensulphide in the presence of an ether.
SO3 + H2S H2S2O3
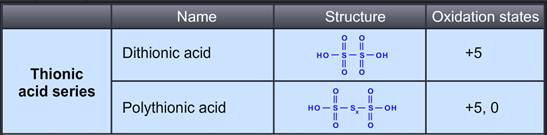
Dithionic acid is moderately strong and stable bibasic acid. It can not be isolated in pure form, only concentrated solutions have been prepared. Dithionates are stable to oxidizing agents and mild reducing agents. On heating, in acid solution, they give both H2SO4 and H2SO3.
H2S2O6 + H2O H2SO4 + H2SO3
Polythionic acid is an oxoacid which has a straight chain of Sulphur atoms.
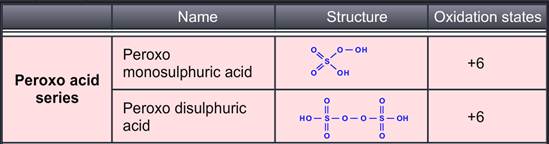
Proxomonosulphuric acid can be produced by adding small quantities of hydrogen peroxide to conc. Sulphuric acid in an inert container. In the anhydrous state, this acid is white crystalline and hygroscopic in nature. It is known as Caro’s acid and used for a variety of disinfectant and cleaning applications. X-ray studies show that peroxodisulphate ion, the structure -O3S-O-O-SO3– with tetrahedral angles at Sulphur atoms. It is also called Marshall’s acid and its salts are powerful oxidizing agents. Also as the acid is prepared from H2O2 and ClSO3H reacting in the molecular ratio 1:2, the structure is linear in nature.
Fig : Polysulphyric acid.
Polysulphyric acid, H2S2O7 can be considered as an anhydride of sulphuric acid. Its structure is the same as one would obtain by combining two molecules of sulphuric acid followed by dehydration of one molecule of water.
Compounds available in pure form are sulphuric acid, disulphuric acid, peroxosulphuric acid, peroxodisulphuric acid, thiosulphuric acid. Except forperoxomonosulphuric acid, all oxoacids of sulphur are dibasic.